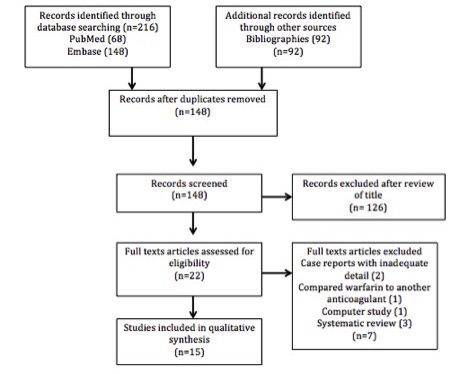
Diseases attributed to tobacco smoking are some of the most prevalent and preventable in the world. Therefore, smoking cessation programs and interventions are crucial components of population health strategies. Currently used interventions and medications have proved effective in aiding patient abstinence from tobacco, yet they are often met with low patient uptake, satisfaction, and compliance. Electronic cigarettes pose a new challenge for clinicians as minimal evidence exists on their safety, health impact and effectiveness as smoking cessation tools.
The evidence to date on electronic cigarettes was reviewed and this guide was developed to assist medical students in providing information and advice to patients about electronic cigarettes. The guide includes information on types of electronic cigarettes, how they work, their health effects, their use in smoking cessation and, current regulation in Australia. The article also includes patient-centred frequently asked questions, with evidence-based answers.

Figure 1: Aerosol from an e-cigarette
Behind the smoke screen
What are e-cigarettes?
Electronic cigarettes, also known as e-cigarettes, e-cigs, personal vaporisers or electronic nicotine delivery systems (ENDS), are battery-operated devices used to simulate the experience of smoking by delivering flavoured nicotine, in the form of an aerosol (Figure 1). Despite the original design dating back to 1963, [1] it was only in 2003 that the Chinese inventor and pharmacist, Hon Lik, was able to develop the first commercially viable modern e-cigarette. [2]
People use e-cigarettes for many reasons, including: To make it easier to reduce the number of cigarettes you smoke (79.0%), they may be less hazardous to your health (77.2%), they are cheaper than regular cigarettes (61.3%), they are a quitting aid (57.8%), so you can smoke in places where smoking regular cigarettes is banned (57.4%), as an alternative to quitting (48.2%), e-cigarettes taste better than regular cigarettes (18.2%). [3]
What makes up an e-cigarette?
There are various classes of e-cigarette, but all follow a simple design. A lithium ion battery is attached to a heating element known as an “atomiser” which vaporises the e-liquid. The e-liquid, sometimes called “juice”, is traditionally held in a cartridge (the mouth piece) and usually consists of a combination of propylene glycol and glycerine (termed humectants) to produce aerosols that simulate conventional cigarette smoke. [4] Liquid nicotine, water, and/or flavourings are commonly included in e-liquids as well. Some devices have a button designed to activate the atomiser; however, more recent designs work via a pressure sensor that detects airflow when the user sucks on the device. This pressure sensor design emits aerosolised vapour, which the user inhales. This practice is known as ‘vaping’.
What types of e-cigarettes exist?
Currently, three classes of e-cigarettes exist on the market [5]:

Figure 2: A cigalike.
“e-Go’s” comprise the second class. These are larger than cigalikes and have removable tanks that can be refilled with e-liquid (Figure 3).

Figure 3: An e-Go.
Finally, there are modular e-cigarettes (or ‘mods’), which are usually larger than e-Go’s. They have a removable tank and can be customised to the user’s preferences (Figure 4).

Figure 4: A modular e-cigarette, or “mod”.
Why is this important?
E-cigarette devices vary vastly between developers. [6] Users are able to modify their e-cigarette atomisers, circuitry, and battery power to alter vapour production. [7-9] By 2014, there were an estimated 466 brands of e-cigarette with 7764 flavours. [10] Users are also able to select their own e-juice, with 97-99% of users choosing e-liquid containing nicotine. [6, 11] Despite devices on the market delivering less nicotine than conventional combustible cigarettes, [12] many health professionals are concerned about the short and long-term health effects of e-cigarettes. [13]
Demystifying the situation
How safe are e-cigarettes?
Given that e-cigarettes have been available for just under a decade, no long-term studies into their health effects currently exist. However, several short-term studies have been conducted on the health implications of e-liquids, e-cigarette devices, and vapour.
Nicotine
The e-cigarette market is largely unregulated. One study found nicotine amounts in e-liquids varied greatly, with concentrations ranging from 0-34 mg/mL. [14] Of additional concern, further studies found significant discrepancies between ‘label concentration’ of nicotine and ‘actual concentration’, [15] with one reporting that ‘nicotine free’ e-liquids actually contained nicotine. [16] This is of ethical concern given that nicotine is a highly addictive drug [17] likely to influence usage patterns and dependence behaviours. There is a need to assess nicotine dependence in e-cigarette users. [18] One study looked at pharmacokinetic absorption of nicotine by comparing nicotine delivery via e-cigarettes, combustion cigarettes, and nicotine inhalers. It found that e-cigarette absorption rates lay between those of combustion cigarettes and nicotine inhalers, implying that nicotine is absorbed though both buccal (slow, nicotine inhaler) and pulmonary (fast, combustion cigarette) routes. As nicotine dependence is related to absorption rate and exposure, this suggests e-cigarettes users are at risk of dependence. This claim was verified by other studies, which conclusively demonstrated e-cigarette users can achieve nicotine exposure similar to that of combustion cigarette smokers. [19,20]
Propylene glycol and glycerine (humectants)
Propylene glycol and glycerine have not been deemed safe for inhalation [21] because little is known about their long-term impacts on health when inhaled. [22] By-products of heating both propylene glycol (propylene oxide) and glycerine (acrolein) have been found to be potentially carcinogenic and irritating to the respiratory tract. [23] A systematic review of contaminants in e-cigarettes concluded that humectants warrant further investigation given the precautionary nature of threshold limit values (TLVs) for exposures to hydrocarbons with no established toxicity (The TLV of a substance being the level to which it is believed a worker can be exposed, day after day, for a working lifetime without adverse health effects). [24]
Flavours
There are over 7000 flavours of e-liquid as of January 2014. Despite nearly all of these flavourings having been approved for human oral consumption, their safety when heated and inhaled remains questionable. [25] In fact, many flavourings have been shown to be cytotoxic when heated and others resemble known carcinogens. [26] One study found heating cinnamon flavoured e-liquid produced cinnamaldehyde, a highly cytotoxic substance, [27] while another study found balsamic flavour e-cigarettes triggered pro-inflammatory cytokine release in lung epithelium. [28] Furthermore, a recent study looking at 30 e-fluids found that the majority of flavours consisted of aldehydes which are known ‘primary irritants’ of the respiratory mucosa. [29] Manufacturers do not always disclose the exact ingredients in their e-liquids and many compounds are potentially cytotoxic, pro-inflammatory and/or carcinogenic. Thus, the safety of e-liquids cannot be assured. [25,30]
Toxins
In the US, the Food and Drug Administration analysed the vapour of 18 cartridges from two leading e-cigarette manufacturers and confirmed the presence of known and potentially carcinogenic or mutagenic substances. These included diethylene glycol (DEG, an ingredient used in antifreeze that is toxic to humans), tobacco-specific nitrosamines (TSNAs, human carcinogens) and tobacco-specific impurities suspected of being harmful to humans (anabasine, myosmine, and β-nicotyrine). [31] To put these findings into context, the concentration of toxins in e-cigarettes ranged between 9 and 450 times less than those in conventional cigarettes. [19] Secondly, they were found to be at acceptable involuntary work place exposure levels. [24] Furthermore, levels of TSNAs were comparable in toxicity to those of nicotine inhalers or patches, [32] two forms of nicotine replacement therapy (NRT) commonly used in Australia. [33] Lastly, e-cigarettes contain only 0.07-0.2% of the TSNAs present in conventional cigarettes. [34] Of note, in 15 subsequent studies that looked at DEG in e-cigarettes, none was found. [34]
E-cigarette device
Many chemicals used in e-liquids are considered safe for oral ingestion, yet their health effects when inhaled as vapour remain uncertain. This applies not only to e-liquids but also the e-cigarette device itself. Many e-cigarette devices are highly customisable, with users able to increase voltages, producing greater toxin levels. One study identified arsenic, lead, chromium, cadmium and nickel in trace amounts not harmful to humans, while another found these elements at levels higher than in combustion cigarettes. [36,37] Lerner et al. looked at reactive oxygen species (ROS) generated in e-cigarette vapour and found them similar to those in conventional smoke. They also found metals present at levels six times greater than in conventional cigarette smoke. [38] A recent review noted that small amounts of metals from the devices in the vapour are not likely to pose a serious health risk to users, [24] while other studies found metal levels in e-cigarette vapour to be up to ten times less than those in some inhaled medicines. [39] Given that metals found in e-cigarette vapour are likely a contaminant of the device [6, 40], variability in the e-cigarette manufacturing process and materials requires stricter regulation to prevent harm to consumers.
Effects on health
E-cigarettes appear to be safer than combustion cigarettes, [15] but they should not be considered harm free. [41] A 2014 Cochrane review found no ‘serious’ adverse effects from e-cigarette trials to date, [42] yet another review which included 28 publications found hazards related to e-cigarettes (Table 1). [43].
Table 1. Frequently reported hazards of electronic cigarette smoking [43]
| Respiratory system |
Upper respiratory tract irritation, dry cough, dryness of the mucus membrane, nose bleeding, release of cytokines and pro-inflammatory mediators, allergic airway inflammation, decreased exhaled nitric oxide (FeNO) synthesis |
| Nervous system |
Headache, dizziness, nervousness, insomnia, sleeplessness |
| GIT |
Nausea, vomiting, dry mouth, mouth or tongue sores/inflammation, black tongue, gum bleeding, gingivitis, gastric burning, constipation |
| CVS |
Palpitation, chest pain |
| Eye |
Irritation, redness and dryness of the eyes, can cause eye damage |
| Choking hazards |
Accidental exposure to high concentrations of e-liquids can cause choking hazards |
| Malignancy |
Change in bronchial gene expression and risk of lung cancer |
| Miscellaneous |
Shortness of breath, shivering etc |
Other large studies supported this information. [23,44-46] Research on short-term changes to cardiorespiratory physiology following e-cigarette use included increased airway resistance [25] and slightly elevated blood pressure and heart rate. [47] As the short- and long-term consequences of e-cigarette use are currently unclear, [47] a conservative stance would be to assume vaping as harmful until more evidence becomes available.
Where there’s smoke, there’s fire
Australian law and e-cigarettes
In Australia there is currently no federal law that specifically addresses the regulation of electronic cigarettes; rather, laws that relate to poisons, tobacco, and therapeutic goods have been applied to e-cigarettes in ways that effectively ban the sale of those containing nicotine. In all Australian states and territories, legislation relating to nicotine falls under the Commonwealth Poisons Standard. [49,50] In all states and territories, the manufacture, sale, personal possession, or use of electronic cigarettes that contain nicotine is unlawful, unless specifically approved, authorised or licenced. [49,50]
Under the Commonwealth Poisons Standard nicotine is considered a Schedule 7 – Dangerous Poison. E-cigarettes containing nicotine could be removed from this category in the future should any device become registered by the Therapeutic Goods Administration (TGA), thus allowing it to be sold lawfully.
There are currently no TGA registered nicotine containing electronic cigarettes [51] and importation, exportation, manufacture and supply is a criminal offence under the Therapeutic Goods Act 1989. [52] It is, however, possible to lawfully import electronic cigarettes containing nicotine from overseas for personal therapeutic use (e.g. as a quitting aid) if one has a medical prescription as this is exempt from TGA registration requirements outlined in the personal importation scheme under the Therapeutic Goods Regulations 1990.
Therefore, it is up to the discretion of the medical practitioner if they provide a prescription for a product not yet approved by the TGA. Given that legislation currently exists to permit medical practitioners to assist individuals in obtaining e-cigarettes, it is imperative we understand both the legal environment at the time and the health consequences.
Stick that in your e-cig and vape it!
E-cigarettes as smoking cessation aids

Figure 5: Quitting tobacco cigarettes through vaping (Image courtesy vaping360.com)
A debate continues as to whether e-cigarettes – with or without nicotine – are able to play a role in smoking cessation (Figure 5). In the absence of large scale clinical trials it is impossible to answer this question definitively. What is clear from smoking statistics worldwide is that more needs to be done regarding smoking cessation. E-cigarettes may be another tool to help achieve a tobacco free future. Thus far, conventional NRT has been rated by most smokers attempting to quit as unappealing [53] despite evidence that NRT increases quit rates by 50-70% compared to placebo. [54] Few trials have been conducted to investigate whether e-cigarettes are effective tools for smoking cessation, but one recent systematic review and meta-analysis found that nicotine containing e-cigarettes were associated with both a significant reduction in the number of combustion cigarettes smoked as well as complete smoking tobacco abstinence. [53] This suggests that e-cigarettes have potential as cessation aids and tobacco harm reduction devices.
E-cigarettes containing nicotine were more successful in helping patients reduce or quit smoking than those without nicotine according to a recent Cochrane review, [42] a finding in-line with conventional NRT vs. placebo studies. The review was unable to compare e-cigarette trials to conventional NRT trials given differences in study designs but commented that on average quit rates using conventional NRT at 12 months were 10%, while e-cigarette use corresponded with quit rates of 20%.
E-cigarettes, unlike conventional NRT products, are not only able to provide smokers with nicotine to satisfy their pharmacological addiction, but by design simulate many of the behaviours that have been psychologically ingrained through long-term smoking. E-cigarettes allow users to inhale and exhale a smoke-like substance. They can handle a device of similar shape to satisfy the oral fixation. Psychological triggers from ‘smoker-friendly venues’ can be relieved by using e-cigarettes, and flavourings can be customised to tobacco or menthol. These factors may prove e-cigarettes a valuable ally in the fight on tobacco. However, there is concern among some health practitioners that e-cigarettes may be a gateway to use of combusted tobacco. [55] If a patient is seeking advice about quitting, it is important to provide them with well tested NRT and medications. These include nicotine delivery preparations for oromucosal (nicotine gum and spray) and transdermal (nicotine patches) routes as well as other drugs including bupropion, varenicline and cytisine medications, [56] with varenicline being the most effective in improving likelihood of quitting. [53]
Questions you may be asked by patients
My partner and I are looking to start a family soon. Is it safe to use electronic cigarettes during pregnancy?
As e-cigarettes lack many of the harmful carcinogens found in regular tobacco cigarettes, consumers might be misled into believing these products are safe. This is of great concern to traditionally high-risk groups, such as pregnant women. In a 2015 review, the author concluded that, based on current evidence, no amount of nicotine is known to be safe during pregnancy. [40] To date, there is no evidence looking specifically at e-cigarette use in pregnant women, however much is known about nicotine exposure in pregnancy. Nicotine is metabolised faster in pregnant women [57] and easily crosses the placental barrier to enter fetal circulation, [40,58] and nicotinic receptors implicated in brain development [59,60] are present in the fetal brain from the first trimester of pregnancy. Many women may seek to use e-cigarettes since conventional NRT in pregnant women has been highly unsuccessful for smoking cessation. [61] Nicotine is considered a Category D drug under Australian pregnancy guidelines (formerly ADEC) and exposure during pregnancy has been found to cause significant health consequences in the fetus and neonate. [62] It is important to inform patients that current evidence suggests nicotine, at any concentration, during pregnancy is not considered safe and all efforts should be made to ensure a nicotine-free pregnancy with effective strategies implemented prior to conception.
My housemates are always using e-cigarettes near me. Can I get sick if I am around them when they use one?
Evidence, especially long-term data, is lacking on the effects of e-cigarettes on bystanders. [13] What is known is e-cigarette vapour contains nicotine and particles that may be inhaled by persons in the vicinity of e-cigarette users. [28] One study found low levels of formaldehyde and nicotine among several other chemicals emitted into the air. It was subsequently concluded that toxins in e-cigarette aerosols were emitted at much lower levels compared with conventional cigarette emissions. [63] A 2014 systematic review [24] compared TLVs to the “worst case” assumptions about both chemical content of aerosols and liquids as well as behaviour of e-cigarette users and concluded “there is no evidence that vaping produces inhalable exposures to contaminants of the aerosol that would warrant health concerns by the standards that are used to ensure safety of workplaces”. Any effect on bystanders from e-cigarette vapour is likely to be much less than combustion cigarettes, a similar conclusion reached by other studies. [39,64] However, some studies have shown serum cotinine levels (the primary metabolite of nicotine) to be increased in non-smokers exposed to e-cigarette vapour, [65,66] though only to levels ten percent of that of second-hand smoke from conventional cigarettes. Even if toxins in vapour are likely to pose little harm to bystanders, the very presence of toxins and nicotine in vapour is inconsistent with the claim most e-cigarette companies make of vapour being ‘just harmless water vapour’.
So I’ve heard e-cigarettes may be unhealthy, but are they dangerous?
There are potential dangers surrounding e-cigarettes arising from their design and engineering. The United States Fire Administration recently compiled a report of over 25 fires or explosions from e-cigarettes, either while being used or charged, many of which resulted in serious burns to individuals and damage to property. [67]
Nicotine in the e-liquid refill packs is considered a potentially lethal poison. [11] If ingested or in direct contact with skin it poses a potential serious health risk, [68] including the potential for overdose in children. [69] There has been at least one known fatality in a toddler from accidental ingestion and overdose of liquid nicotine intended for e-cigarette use. There have been over 3500 liquid nicotine exposure related incidents recorded by the American Association of Poison Control Centres since November 2014. [70]
What are tobacco companies doing about e-cigarettes?
It is worth noting that many tobacco companies have opted to include e-cigarettes in their product portfolio. [6] Thus ethically speaking, it is vital for doctors to understand that by recommending e-cigarettes they may indirectly be supporting the tobacco industry.
Conclusion
E-cigarettes are a growing market and present a novel challenge to clinicians and medical students. Traditional approaches of obtaining pack-year histories or relying on tell-tale signs of smoking such as tar stained fingers or smoke odour will not work for e-cigarette users. We must ask specifically about use of e-cigarettes when taking a smoking history, use terms like ‘vaping’, ask whether the e-juice contains nicotine and if they have customised their devices. We must not become complacent simply because e-cigarettes are currently viewed as the lesser of two evils with regards to impact on health. As medical students, deciding whether or not to endorse e-cigarettes as smoking cessation aids is a complex issue given that proven, safe, and effective treatments currently exist, and those should be used as primary cessation aids. If a patient has used these primary aids and failed to quit, it is worthwhile considering e-cigarettes as an avenue for achieving tobacco abstinence. It is unlikely that the clinicians we encounter in our studies will have a detailed understanding on e-cigarettes and vaping practises; it is therefore up to us to keep abreast of such knowledge to provide patients with quality information and care.
Acknowledgments
The author would like to thank Kelly Mirowska-Allen, a third-year medical student at the University of Melbourne, for assistance in proofreading this article and providing support and advice.
Conflict of interest
None declared.
References
[1] A GH. Smokeless non-tobacco cigarette. Google Patents; 1965.
[2] Hancock T. Chinese e-cigarette inventor fights for royalties. The Japan Times [Internet]. 2013 Oct 4 [cited 2015 27th July]. Available from: http://www.japantimes.co.jp/news/2013/10/04/business/chinese-e-cigarette-inventor-fights-for-royalties/#.VbXikPmqqkq.
[3] Hummel K, Hoving C, Nagelhout GE, de Vries H, van den Putte B, Candel MJ, et al. Prevalence and reasons for use of electronic cigarettes among smokers: Findings from the International Tobacco Control (ITC) Netherlands Survey. Int J Drug Policy. 2015;26(6):601-8.
[4] Cheng T. Chemical evaluation of electronic cigarettes. Tob Control. 2014;23 Suppl 2:ii11-7.
[5] Farsalinos KE, Romagna G, Tsiapras D, Kyrzopoulos S, Voudris V. Characteristics, perceived side effects and benefits of electronic cigarette use: a worldwide survey of more than 19,000 consumers. Int J Environ Res Public Health. 2014;11(4):4356-73.
[6] Born H, Persky M, Kraus DH, Peng R, Amin MR, Branski RC. Electronic Cigarettes: A Primer for Clinicians. Otolaryngol Head Neck Surg. 2015;153(1):5-14.
[7] Cooper S. What you need to know about vaporizers: Engadget [Internet]. 2014 May 23 [cited 2015 28 July]. Available from: http://www.engadget.com/2014/05/23/vaporizers-explainer/.
[8] Larson E. Pimp My Vape: The rise of e-cigarette hackers. Mashable Australia [Internet]. 2014 Jan 25 [cited 2015 28 July]. Available from: http://mashable.com/2014/01/25/vaping-subculture/.
[9] Ebbert JO, Agunwamba AA, Rutten LJ. Counseling patients on the use of electronic cigarettes. Mayo Clin. Proc. 2015;90(1):128-34.
[10] Zhu SH, Sun JY, Bonnevie E, Cummins SE, Gamst A, Yin L, et al. Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tob Control. 2014;23 Suppl 3:iii3-9.
[11] Brandon TH, Goniewicz ML, Hanna NH, Hatsukami DK, Herbst RS, Hobin JA, et al. Electronic nicotine delivery systems: a policy statement from the American Association for Cancer Research and the American Society of Clinical Oncology. Clin. Cancer Res. 2015; 21(3):514-25.
[12] Farsalinos KE, Spyrou A, Tsimopoulou K, Stefopoulos C, Romagna G, Voudris V. Nicotine absorption from electronic cigarette use: comparison between first and new-generation devices. Sci Rep. 2014;4:4133.
[13] Callahan-Lyon P. Electronic cigarettes: human health effects. Tob Control. 2014;23 Suppl 2:ii36-40.
[14] Goniewicz ML, Hajek P, McRobbie H. Nicotine content of electronic cigarettes, its release in vapour and its consistency across batches: regulatory implications. Addiction. 2014;109(3):500-7.
[15] Farsalinos KE, Polosa R. Safety evaluation and risk assessment of electronic cigarettes as tobacco cigarette substitutes: a systematic review. Ther Adv Drug Saf. 2014;5(2):67-86.
[16] Goniewicz ML, Knysak J, Gawron M, Kosmider L, Sobczak A, Kurek J, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. 2014;23(2):133-9.
[17] Nestler EJ. Cellular basis of memory for addiction. Dialogues Clin Neurosci. 2013;15(4):431-43.
[18] Fagerstrom K, Eissenberg T. Dependence on tobacco and nicotine products: a case for product-specific assessment. Nicotine Tob Res. 2012;14(11):1382-90.
[19] Schroeder MJ, Hoffman AC. Electronic cigarettes and nicotine clinical pharmacology. Tob Control. 2014;23 Suppl 2:ii30-5.
[20] Dawkins L, Corcoran O. Acute electronic cigarette use: nicotine delivery and subjective effects in regular users. Psychopharmacology. 2014;231(2):401-7.
[21] Jimenez Ruiz CA, Solano Reina S, de Granda Orive JI, Signes-Costa Minaya J, de Higes Martinez E, Riesco Miranda JA, et al. The electronic cigarette. Official statement of the Spanish Society of Pneumology and Thoracic Surgery (SEPAR) on the efficacy, safety and regulation of electronic cigarettes. Arch Bronconeumol. 2014;50(8):362-7.
[22] Pepper JK, Brewer NT. Electronic nicotine delivery system (electronic cigarette) awareness, use, reactions and beliefs: a systematic review. Tob Control. 2014;23(5):375-84.
[23] Grana R, Benowitz N, Glantz SA. E-cigarettes: a scientific review. Circulation. 2014;129(19):1972-86.
[24] Burstyn I. Peering through the mist: systematic review of what the chemistry of contaminants in electronic cigarettes tells us about health risks. BMC Public Health. 2014;14:18.
[25] Bertholon JF, Becquemin MH, Annesi-Maesano I, Dautzenberg B. Electronic cigarettes: a short review. Respiration. 2013;86(5):433-8.
[26] Bahl V, Lin S, Xu N, Davis B, Wang YH, Talbot P. Comparison of electronic cigarette refill fluid cytotoxicity using embryonic and adult models. Reprod Toxicol. 2012;34(4):529-37.
[27] Behar RZ, Davis B, Wang Y, Bahl V, Lin S, Talbot P. Identification of toxicants in cinnamon-flavored electronic cigarette refill fluids. Toxicol In Vitro. 2014;28(2):198-208.
[28] Rom O, Pecorelli A, Valacchi G, Reznick AZ. Are E-cigarettes a safe and good alternative to cigarette smoking? Ann. N. Y. Acad. Sci. 2015;1340:65-74.
[29] Tierney PA, Karpinski CD, Brown JE, Luo W, Pankow JF. Flavour chemicals in electronic cigarette fluids. Tob Control. 2015.
[30] Schraufnagel DE, Blasi F, Drummond MB, Lam DC, Latif E, Rosen MJ, et al. Electronic cigarettes. A position statement of the forum of international respiratory societies. Am J Respir Crit Care Med. 2014;190(6):611-8.
[31] FDA. Public Health Focus – Summary of Results: Laboratory Analysis of Electronic Cigarettes Conducted By FDA [WebContent]. 2015 [cited 2015 28 July]. Available from: http://www.fda.gov/NewsEvents/PublicHealthFocus/ucm173146.htm.
[32] Stepanov I, Jensen J, Hatsukami D, Hecht SS. Tobacco-specific nitrosamines in new tobacco products. Nicotine Tob Res. 2006;8(2):309-13.
[33] Quit Victoria. Nicotine replacement products 2015 [cited 2015 28 July]. Available from: http://www.quit.org.au/preparing-to-quit/choosing-best-way-to-quit/nicotine-replacement-products.
[34] Cahn Z, Siegel M. Electronic cigarettes as a harm reduction strategy for tobacco control: a step forward or a repeat of past mistakes? J Public Health Policy. 2011;32(1):16-31.
[35] Chausse P, Naughton G, Dutheil F. Electronic Cigarettes: The Resistance Value of the Heating Filament Could Be the Key to Lung Toxicity. Chest. 2015;148(1):e29-30.
[36] Cobb NK, Abrams DB. E-cigarette or drug-delivery device? Regulating novel nicotine products. N Engl J Med. 2011;365(3):193-5.
[37] Williams M, Villarreal A, Bozhilov K, Lin S, Talbot P. Metal and silicate particles including nanoparticles are present in electronic cigarette cartomizer fluid and aerosol. PloS one. 2013;8(3):e57987.
[38] Lerner CA, Sundar IK, Watson RM, Elder A, Jones R, Done D, et al. Environmental health hazards of e-cigarettes and their components: Oxidants and copper in e-cigarette aerosols. Environ Pollut. 2015;198:100-7.
[39] Hajek P, Etter JF, Benowitz N, Eissenberg T, McRobbie H. Electronic cigarettes: review of use, content, safety, effects on smokers and potential for harm and benefit. Addiction. 2014;109(11):1801-10.
[40] Suter MA, Mastrobattista J, Sachs M, Aagaard K. Is there evidence for potential harm of electronic cigarette use in pregnancy? Birth Defects Res A Clin Mol Teratol. 2015; 103(3):186-95.
[41] Pisinger C, Dossing M. A systematic review of health effects of electronic cigarettes. Prev Med. 2014;69:248-60.
[42] McRobbie H, Bullen C, Hartmann-Boyce J, Hajek P. Electronic cigarettes for smoking cessation and reduction. Cochrane Database Syst Rev. 2014;12:CD010216.
[43] Meo SA, Al Asiri SA. Effects of electronic cigarette smoking on human health. Eur Rev Med Pharmacol Sci. 2014;18(21):3315-9.
[44] Gualano MR, Passi S, Bert F, La Torre G, Scaioli G, Siliquini R. Electronic cigarettes: assessing the efficacy and the adverse effects through a systematic review of published studies. J Public Health. 2014.
[45] Franck C, Budlovsky T, Windle SB, Filion KB, Eisenberg MJ. Electronic cigarettes in North America: history, use, and implications for smoking cessation. Circulation. 2014;129(19):1945-52.
[46] Hua M, Alfi M, Talbot P. Health-related effects reported by electronic cigarette users in online forums. J Med Internet Res. 2013;15(4):e59.
[47] Orellana-Barrios MA, Payne D, Mulkey Z, Nugent K. Electronic Cigarettes-A Narrative Review for Clinicians. Am J Med. 2015;128(7):674-81.
[48] Chapman S, Byrne F, Carter SM. “Australia is one of the darkest markets in the world”: the global importance of Australian tobacco control. Tob Control. 2003;12 Suppl 3:iii1-3.
[49] Victoria Q. Legal status of electronic cigarettes in Australia. 2015 [cited 2015 26 July]. Available from: http://www.quit.org.au/downloads/resource/policy-advocacy/policy/legal-status-electronic-cigarettes-australia.pdf.
[50] The Poisons Standard (the SUSMP) | Therapeutic Goods Administration (TGA). 2015 [cited 2015 26 July]. Available from: https://www.tga.gov.au/publication/poisons-standard-susmp.
[51] TGA. Electronic cigarettes | Therapeutic Goods Administration (TGA) 2015 [cited 2015 26 July]. Available from: http://www.tga.gov.au/community-qa/electronic-cigarettes.
[52] Therapeutic Goods Act Section 19B, (1989).
[53] Rahman MA, Hann N, Wilson A, Mnatzaganian G, Worrall-Carter L. E-cigarettes and smoking cessation: evidence from a systematic review and meta-analysis. PloS one. 2015;10(3):e0122544.
[54] Stead LF, Perera R, Bullen C, Mant D, Hartmann-Boyce J, Cahill K, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2012;11:CD000146.
[55] Klein JD. Electronic Cigarettes Are Another Route to Nicotine Addiction for Youth. JAMA Pediatr. 2015:1-2.
[56] Green G. Nicotine Replacement Therapy for Smoking Cessation. Am Fam Physician. 2015;92(1):Online.
[57] Dempsey D, Jacob P, 3rd, Benowitz NL. Accelerated metabolism of nicotine and cotinine in pregnant smokers. J Pharmacol Exp Ther. 2002;301(2):594-8.
[58] Luck W, Nau H, Hansen R, Steldinger R. Extent of nicotine and cotinine transfer to the human fetus, placenta and amniotic fluid of smoking mothers. Dev Pharmacol Ther. 1985;8(6):384-95.
[59] Hellstrom-Lindahl E, Nordberg A. Smoking during pregnancy: a way to transfer the addiction to the next generation? Respiration. 2002;69(4):289-93.
[60] Nasrat HA, Al-Hachim GM, Mahmood FA. Perinatal effects of nicotine. Biol Neonate. 1986;49(1):8-14.
[61] Coleman T, Chamberlain C, Cooper S, Leonardi-Bee J. Efficacy and safety of nicotine replacement therapy for smoking cessation in pregnancy: systematic review and meta-analysis. Addiction. 2011;106(1):52-61.
[62] Bruin JE, Gerstein HC, Holloway AC. Long-term consequences of fetal and neonatal nicotine exposure: a critical review. Toxicol Sci. 2010;116(2):364-74.
[63] Schripp T, Markewitz D, Uhde E, Salthammer T. Does e-cigarette consumption cause passive vaping? Indoor Air. 2013;23(1):25-31.
[64] McAuley TR, Hopke PK, Zhao J, Babaian S. Comparison of the effects of e-cigarette vapor and cigarette smoke on indoor air quality. Inhal Toxicol. 2012;24(12):850-7.
[65] Drummond MB, Upson D. Electronic cigarettes. Potential harms and benefits. Ann Am Thorac Soc. 2014;11(2):236-42.
[66] Czogala J, Goniewicz ML, Fidelus B, Zielinska-Danch W, Travers MJ, Sobczak A. Secondhand exposure to vapors from electronic cigarettes. Nicotine Tob Res. 2014;16(6):655-62.
[67] Administration USF. Electronic Cigarette Fires and Explosions 2014 [cited 2015 30 July]. Available from: https://www.usfa.fema.gov/downloads/pdf/publications/electronic_cigarettes.pdf.
[68] Yang L, Rudy SF, Cheng JM, Durmowicz EL. Electronic cigarettes: incorporating human factors engineering into risk assessments. Tob Control. 2014;23 Suppl 2:ii47-53.
[69] Bhatnagar A, Whitsel LP, Ribisl KM, Bullen C, Chaloupka F, Piano MR, et al. Electronic cigarettes: a policy statement from the American Heart Association. Circulation. 2014;130(16):1418-36.
[70] Gillian Mohney. First Child’s Death From Liquid Nicotine Reported as ‘Vaping’ Gains Popularity. ABC News [Internet]. 2014 Dec 12 [cited 2015 30 July]. Available from: http://abcnews.go.com/Health/childs-death-liquid-nicotine-reported-vaping-gains-popularity/story?id=27563788.
.jpg?itok=ia0FVI2-)