Opioids and benzodiazepines are frequently prescribed medications that carry a high risk of dependency and are commonly misused. Long-term use of these agents is associated with significant harm to the individual such as exacerbating the patient’s original symptoms, increasing mortality, and negatively impacting the community by contributing to road trauma. This paper will begin by outlining the regulation of drugs of dependence in Australia and defining misuse, dependency, and associated harms. The evidence for use of these drug classes will then be presented, with reference to best practice guidelines. Finally, the discrepancy between guidelines and prescribing behaviours will be highlighted with reference to specific challenges associated with drugs of dependency. It will be emphasised that doctors, especially the newer generations, have a responsibility to own the problems of drug misuse, and to impact individuals and the community through advocacy and evidence-based clinical governance. It will be shown that whilst opioids and benzodiazepines are effective short-term medicines, there is little support for long-term prescribing. If we are to turn the tide on drug dependency and misuse, change is needed in modern prescribing behaviours to achieve the best long-term outcomes for patients.
The problem
The most commonly misused prescription drugs in Australia are opioids and benzodiazepines, including z-drugs (zolpidem, zopiclone, and zalephon), which are non-benzodiazepines that act on the same receptors [1-3]. Access to these drugs is restricted by the Australian Therapeutic Goods Association Drug Schedule, which determines availability for public consumption [4]. Drugs of dependency include all Schedule 8 drugs (controlled drugs, such as opioids) and some Schedule 4 drugs (prescription only medicines, such as benzodiazepines) [5]. Such restrictions are regularly reviewed and subject to change. For example, alprazolam, a potent benzodiazepine with high toxicity [6], was made Schedule 8 in early 2014 due to a rise in illicit use [7], and there is current debate about the appropriateness of Schedule 4 for codeine, because of increasing codeine-related deaths [8,9].
Drug misuse is an important umbrella term to define in relation to the problems associated with drugs of dependency. Five main categories, that are not mutually exclusive, emerge in the literature:
- Overuse: Higher dose or dose frequency than prescribed; often due to tolerance and self-adjusted dosing over long-term use [3].
- Abuse: Overuse with the goal of intoxication [3].
- Prolonged use: An emerging problem involving continued therapy to achieve a sense of normality. Such individuals have been termed the “hidden population” [2-3]. Similarly, the term “accidental addicts” has emerged in popular media, referring to individuals who become dependent on pharmaceuticals with continued use. Prescribers must be hypervigilant for these patients as they are often of high socioeconomic status and highly functional, in contrast to traditional drug-seeking stereotypes [10].
- Substance use disorder: This is a formal diagnosis in the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5) based on eleven symptomatology criteria across four categories (impaired control, social impairment, risky use and pharmacology) [11]. For diagnosis, use must cause clinically significant distress or impairment and occur within a twelve-month period [11].
- Dependency: A syndrome in which an individual has: (i) a strong drive to use a substance; (ii) difficulty regulating their use; (iii) tolerance; and (iv) withdrawal symptoms on cessation [12].
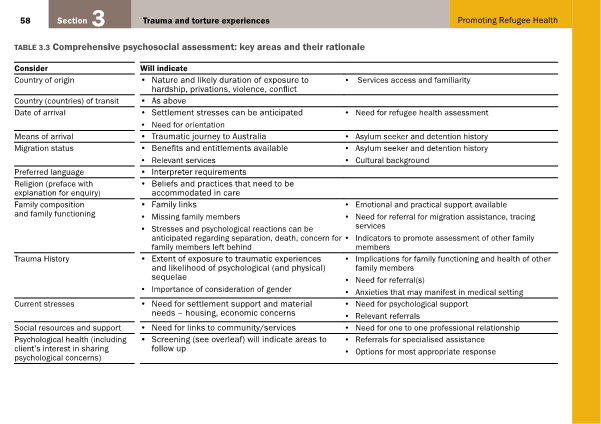
Opioids and benzodiazepines are included on the World Health Organization list of essential medicines [13], indicated primarily in pain or palliative care (opioids), and anxiety disorders, seizures, acute insomnia, and alcohol withdrawal (benzodiazepines) [3]. Availability of opioids and benzodiazepines has been steadily increasing in recent decades. The number of opioid-dispensing episodes increased from 500,000 to 7.5 million from 1992 to 2012 [14]. Harrison and colleagues evaluated 4666 encounters with general practitioners (GPs) between 2010 and 2011 in which opioids were prescribed or supplied [15]. They found that 3.5% of opioids were prescribed for cancer pain, 43.9% were prescribed for chronic non-cancer pain, with the remainder being prescribed for indications classified as non-chronic [15]. For benzodiazepines, approximately 7 million scripts are written annually [16]. The 2013 National Drug Strategy Household Survey found that 3.3% of people aged 14 or over had used analgesics and 1.6% had used tranquillisers or sleeping pills over the past year for non-medical purposes, with both increasing over each three-yearly iteration of the survey since 2001 [17]. With problematic usage and availability of these drugs in the community (through either personal prescription or diversion), the harm associated with misuse has become increasingly apparent. There is a great degree of harm caused by opioid and benzodiazepine misuse to the individual and community (Table 1).
Table 1: Harms to the individual and community associated with opioid and benzodiazepine misuse
| Harms |
Opioids |
Benzodiazepines |
|
Individual |
High risk of dependence [18] and misuse [2] |
Extremely habit forming: tolerance and dependence can occur in 2-4 weeks [31-33] |
| Physiological effects
Constipation [19]
Biliary dyskinesia [19]
Cognitive impairment [19]
Increased risk of death [20]/overdose (including inadvertent childhood ingestion) [2]
Sleep apnoea [21]
Sexual and endocrine dysfunction [22]
Immunosuppression [23]
Opioid-induced hyperalgesia [24]
Osteoporosis/increased fracture risk [2]
Dental caries and necrosis secondary to xerostomia [2]
Impaired cognition [2] |
Physiological effects
Insomnia [7]
Anxiety [7]
Depression [34]
Poor memory and concentration [35]
Excessive / daytime sedation [36]
Risk of ataxia and falls, disinhibition and amnestic effects [36]
Cognitive impairment [37]/decline and dementia [38]
Increased mortality with long term use [39]
|
| Codeine: gastric bleeding [25], severe metabolic imbalances [26-28], and risk of death [8] |
Z-drugs: potentially dangerous complex sleep behaviours [16]
|
| Harms with intravenous abuse: blood clots, amputation, and serious soft tissue injuries [29,30] |
Harms with intravenous abuse: blood clots, amputation, and serious soft tissue injuries [29,30] |
| Community |
Diversion [40]
Low levels of organised crime [41]
Driving impairment and motor vehicle accidents [42,43]
Doctor shopping affecting GPs [3]
|
Diversion [7]
Low levels of organised crime [41]
Driving impairment and motor vehicle accidents [42,43]
Doctor shopping impacting GPs [3]
Criminal activity through precipitating aggression and disinhibition [41] |
Opioids and benzodiazepines are highly habit-forming and potentially destructive to the individual and society. Physiological side effects of long-term use of both drug classes significantly reduce quality of life and contribute to personal suffering through symptoms such as constipation and balance derangement contributing to falls. Further, opioids and benzodiazepines are both associated with increased mortality. This is through means such as deliberate overdose, accidental overdose, and motor vehicle accidents secondary to impaired driving ability [2,42,43]. In Victoria, overdose deaths involving prescription drugs outnumber deaths from road trauma [44], and alcohol and drug helplines receive nearly threefold more calls regarding prescription opioids than heroin [45]. Importantly, an Australian study investigating 320 oxycodone related deaths reported that 52% were due to unintentional overdose, 20% were deliberate self-harm, and the remainder either awaiting coronial enquiry or with indeterminable intent [46]. Victorian coronial data indicate that benzodiazepines are at least contributory in 48.8% of all drug-related deaths [47]. As such, long-term opioid and benzodiazepine use is associated with a very real increase in mortality, not only in people with the intent to self-harm. Overall, opioids and benzodiazepines carry a high risk of dependence, cause a wide range of physiological harms including increased mortality, and they negatively affect the community through diversion and contributing to motor vehicle accidents.
The evidence
Current evidence supports short-term use of both opioids and benzodiazepines for appropriate indications (for example, less than 90 days and one month, respectively). These drugs are effective when used properly and should be available for people who need them. Long-term use, however, is not supported by evidence for the indications for short-term use. Both classes of medications are known to be highly addictive and associated with poor long-term outcomes if use is not time limited. Notably, recent studies suggest that psychobehavioural interventions are at least equivalent to, or more effective than, pharmacotherapy for pain, anxiety, and insomnia [48-50].
Opioids have convincing evidence supporting their use in acute pain [51], cancer pain [52], palliative care, [53] and addiction [54]. Most of the controversy in opioid prescribing relates to use in chronic non-cancer pain, with reviews suggesting little short-term efficacy [55], and tolerance and opioid-induced hyperalgesia occurring with prolonged use [56]. Furthermore, a Cochrane Database review showed no clear gains in pain and functional outcomes when opioids were used long-term [57], and another study found no significant correlations between opioid dose adjustment and clinical pain scores over time [58]. In line with the biopsychosocial conceptualisation of chronic non-cancer pain, multimodal approaches using non-pharmacological strategies such as exercise and psychological interventions have been shown to be superior to monomodal regimens in terms of long-term impact [59]. Cognitive behavioural therapy (CBT) is recommended as first line psychosocial treatment for chronic, non-cancer pain [60]. A Cochrane review in 2012 found that CBT conferred significant improvement in pain, disability, mood, and catastrophising in comparison to wait-list controls [61]. Effect sizes were small to moderate and the only sustained impacts were seen on mood.
Short acting benzodiazepines and z-drugs are effective at managing acute insomnia [62], generalised anxiety disorder, social anxiety, and panic disorder [63]. Dependency can be rapid (for example, within 2 to 4 weeks) [64] and increased mortality has been associated with long-term use [39]. Unfortunately, randomised controlled trials for long-term use are of inadequate duration (approximately 3 months), and lack representative clinical samples [16]. Reassuringly, however, it has been shown that the majority of patients on benzodiazepine therapy for sleep and anxiety do not increase their doses over time [65]. Moreover, a recent three year follow-up study of users of benzodiazepines and z-drugs found less than 1% of patients developed problems with excessive use, which predominated in patients with a significant drug and alcohol history and increased with duration of therapy [66]. This suggests that careful evaluation of patients to identify those at risk of excessive use is important, and that monitoring and cessation plans at first prescription may be protective for this population. Of note, this paper did not assess experience of adverse effects or harms associated with long-term use of benzodiazepines. Instead, it focussed on excessive use only, defined as more than two daily doses over a three month period.
Insomnia treatment is generally recommended to proceed in a stepwise fashion, with initial management emphasising basic sleep hygiene education and stimulus control, with the latter supported by randomised controlled trial evidence for long-term efficacy [67]. If insomnia persists, more involved behavioural interventions, either with or without medications, should be offered. CBT combines sleep hygiene education, stimulus control, and progressive muscle relaxation into a cognitive therapy framework to address insomnia. A recent meta-analysis of randomised controlled trials has shown lasting benefits for establishing sleep, wake times, and sleep efficiency [68]. However, total sleep time was not significantly increased and the patient samples excluded those with medical and psychological comorbidities. As such, the magnitude of the effects needs to be interpreted with caution. CBT is recommended as first line treatment for insomnia by the Royal Australian College of General Practitioners (RACGP) and the British Association for Pharmacology [69] on the basis of previous systematic reviews, which have included randomised controlled trials and patients with medical and psychiatric comorbidities and shown reliable and sustained improvements on sleep parameters [70]. Similarly, in the treatment of anxiety disorders, CBT is supported by meta-analysis of randomised controlled trials [71] and is recommended as first line therapy, either as monotherapy or in combination with antidepressant medication [72].
Current guidelines are in line with the evidence base for long-term prescribing of opioids and benzodiazepines in Australia. The RACGP published two comprehensive documents in 2015 about prescribing drugs of dependency (with particular reference to benzodiazepines) that summarise best evidence, provide concrete guidance and examples of difficult consultations, and ways in which they can be safely and effectively negotiated [5,16]. For opioid prescribing in chronic, non-cancer pain, the Hunter Integrated Pain Service provides additional evidence-based guidelines [19]. An overview of the key prescribing guidelines can be extracted from these documents (Table 2).
Table 2: Summary of guidance for opioid and benzodiazepine prescribing from Australian evidence-based guidelines
| Guideline |
Authors |
Summary of recommendations |
|
Prescribing drugs of dependence in general practice, part A – clinical governance framework* [5] |
RACGP |
- Maintain and develop skills in chronic pain, mental health, drugs of dependency (DOD) and optimise non-pharmacological interventions
- Inform patients DOD should be prescribed from one practice, one GP and dispensed from one pharmacy
- In complex cases or if situations deteriorate, be prepared to utilise specialist support
- Prescribing for drug dependent patients must be based on comprehensive assessment and an authority must be sought when prescribing a Schedule 8 drug
- Patients have the right to respectful care that promotes their dignity, privacy, and safety
- Provide patients with information about the purpose, realistic expectations, options, and benefits and risks
- Develop respectful, non-judgemental, and clear responses to inappropriate requests for DOD
|
| Prescribing drugs of dependence, part B – benzodiazepines[16] |
RACGP |
- Prescription of benzodiazepines (BZDs) should be with the lowest dose and shortest possible timeframe, continual monitoring, and careful consideration of risks and benefits
- Discuss with patients the potential for dependence, withdrawal, misuse, and known harmful effects such as falls, cognitive decline, and motor vehicle accidents
- Avoid prescribing to patients with comorbid alcohol or substance use disorders (these patients are more vulnerable to major harms)
- Use beyond four weeks should be uncommon, if no alternatives are available, long-term use must be supervised with regular attempts at reduction
- For insomnia, first line should be non-pharmacological (e.g. CBT) and BZDs/Z-drugs only given on an individual basis, with short-term, intermittent dosing
- For anxiety disorders, first line therapy should include CBT; SSRI/SNRI are suitable first line pharmacological treatments
- BZD use in anxiety disorders is mostly limited to severe or treatment resistant cases
- Short term BZD use as occasional adjunctive therapy may be effective at reducing symptoms in the first few days to weeks of SSRI/SNRI therapy
- BZD discontinuation can be achieved with minimal interventions (such as GP advice/advisory letters), strong therapeutic alliance and psychological therapies
|
| Reconsidering opioid therapy: a Hunter New England perspective [19] |
Hunter Integrated Pain Service |
- Opioid therapy is indicated for acute pain; cancer pain, palliative care; opioid dependency/addiction – NOT chronic, non-cancer pain
- Multidimensional pain assessment is recommended for all types of pain
- A drug and alcohol history, use of the risk assessment tools to gauge the risk of opioid misuse and contact with the Australian Prescription Shopping Information service is recommended
Opioid therapy for acute pain:
- Should be time limited and coordinated between hospital and primary care
- Can usually be ceased within one week of surgery or injury if possible, and weaned and ceased within 90 days in complex cases
- A daily oral morphine equivalent dose of 100 mg should not be exceeded in primary care
- A treatment agreement explaining potential benefits, adverse effects, and duration of therapy should be used, with pain and functional outcomes measured
- Review therapy regularly with the “four A’s”: Analgesia, Activity, Adverse effects, and Aberrant behaviour
- Should be prescribed with ongoing non-pharmacological supportive care and a focus on evidence-based self-management strategies
- Involvement of a pain medicine specialist and multidisciplinary pain management team may be helpful
- Opioid naïve patients prescribed opioids and patients on long term opioids and BZDs should not drive
|
*These guidelines also provide recommendations for clinical governance and general practice systems of care not summarised here.
The need for evidence-based prescribing behaviours
Evidence does not support long-term therapy with opioids and benzodiazepines. This has been known for some years, yet the problem has only grown. Future generations of practitioners need to engender practice in accordance with guidelines, enhance utilisation of alternative, non-pharmacological interventions, and strive for better patient outcomes.
Consultations that involve the consideration of drugs of dependence present significant challenges to the GP, and usually involve complex and vulnerable patients. GPs report complicating factors such as time pressure, concerns about patient autonomy, patient-centred care and preserving the doctor-patient relationship [73-75]. Studies have shown that GPs have some ambivalence towards prescribing benzodiazepines and may lack consistency in their approach [73]. There is also referral to the notion of the “deserving patient” in the literature, whereby doctors ask themselves whether the patient deserves a prescription rather than whether they should write one [76]. Indeed, it has been reported that some GPs will provide benzodiazepines even when they do not believe it will help, giving reasons such as: having no realistic alternatives; assumptions about the patients’ expectations; problems in saying no; and succumbing to pressure [16]. Importantly, research indicates that many doctors assume that patients want prescriptions or would resist attempts at withdrawal whilst, in fact, patients report wanting explanations and discussion [73,77].
Inevitably, there is often a discrepancy between clinical guidelines and practice. There is some disagreement in the literature regarding the usefulness and clinical impact of best practice guidelines, with some findings demonstrating improved and more consistent management [78,79], whilst others show minimal impacts [80]. Recent studies are suggesting that GPs perceive clinical practice guidelines with a positive attitude [81], but that there are inconsistencies regarding which components are followed [82]. Research into the appropriate prescribing of antibiotics suggests that inappropriate prescribing is more likely to occur in high-volume practices and by those who have been in practice longer [83]. As such, to improve concordance with guidelines, it will be important for junior doctors to emerge into the workplace as proponents of evidence-based prescribing. They should take advantage of their training period, where case loads are lower, to ingrain safe prescribing habits and good clinical governance into their practice. Obviously, there is more to appropriate prescribing than simply following guidelines. There is an art to handling challenging consultations, conveying evidence in a meaningful way, inspiring trust in your patient, and establishing a collaborative management plan, which may or may not meet the patient’s original expectations. In light of this, there are some additional consultation techniques that may facilitate safe prescribing for junior doctors (Table 3).
Table 3: Practical advice for managing patients and drugs of dependency
| Areas of Development |
Strategies |
|
Professional Knowledge |
Engage in continuing medical education for drug dependence and safe prescribing
Develop skills in cognitive behavioural strategies and structured problem solving [84,85]
Familiarise with typical patient requests and responses from examples [5,16] |
|
Consultation Skills
Time spent in early consultations will save time long-term
|
Encourage non-pharmacological measures
Assess risk/benefits of any drug prescribed
Make collaborative decision making for prescribing and non-prescribing [5]
Prescribe only for supported indications
Prescribe with plan for reduction [5]
Be honest and explore issues [5]
Involve the patient’s supports [5]
Monitor use and look for signs of dependency (review ‘five As’) [5]
Identify problem use and refer to specialist services [5]
Provide written management plan and fact sheets [5]
Seek advice if unsure |
| Resources |
Withdrawal scales for alcohol [86], opioids [87], and benzodiazepines [88]
Therapeutic Guidelines for withdrawal management
Opioid risk tool [89]
Specialist drug and alcohol services
NPS MedicineWise Key Points for managing chronic pain [90]
The patient’s pharmacist [91]
Local support groups
Medicare Australia prescription shopping information service (ph. 1800 631 181) [2] |
Junior doctors and emerging medical professionals have the power to be agents of change in the evidence-based management of conditions such as chronic, non-cancer pain; anxiety; and insomnia. Doctors in training are often the most up-to-date on evidence-based guidelines in these areas, with greater emphasis on non-pharmacological management and multidisciplinary collaboration in undergraduate and postgraduate programs than in generations past. In addition, medical school curriculums have been modified to promote highly developed communication skills in graduates that are crucial to the ability to inspire motivational change in patients. As highlighted in Table 3, doctors can up-skill by developing their professional knowledge and consulting skills, and engaging with the wealth of resources available to them. Continuing medical education is paramount and skills in cognitive behavioural strategies such as goal setting and structured problem solving, mindfulness strategies, and motivational interviewing are vital to being an effective clinician. In addition, the RACGP provides examples of consultations and scripted responses to common questions such as “I need something to help me sleep” or “I want medication”, which are helpful for the emerging practitioner whilst they are still building clinical experience [16]. In the consulting room, the junior doctor can advocate evidence-based, non-pharmacological approaches; conduct appropriate risk assessment for dependence; and prescribe appropriately with reduction plans already in place. The ‘five As’ of pain medicine (Analgesia, Activity, Adverse effects, Aberrant behaviour, and Affect) provide a useful framework for assessing risk of dependence [5].
It is best practice to seek advice when there is suspicion of problematic use, and link patients in with specialist services. Managing patients who are substance dependent is highly challenging. Leveraging support organisations, specialist alcohol and drug services, community pharmacists, and prescription tracking services provides greater likelihood of positive patient outcomes. Furthermore, it will protect the primary medical professional from burnout.
In order to improve patient outcomes, doctors need to begin raising patient awareness of the likelihood and seriousness of adverse effects of these medications, and enthusiastically advocate and facilitate evidence-based treatments. Moreover, the right treatments need to be provided for the right indications. This is, after all, at the heart of patient-centred care, rather than allowing motivated and vulnerable patients to choose their own treatment.
Conclusion
While current prescribing behaviours are understandable because of patient expectations and time pressure, particularly limiting the opportunity for patient education, this is an area that will need to be addressed in the current generation of training doctors. Guidelines to assist in this have been provided in this paper along with some practical strategies that can be implemented to drive the changes necessary to improve patients’ long-term outcomes. Whilst opioids and benzodiazepines are drugs of dependency, neither the doctor nor the patient should depend on these drugs to treat a long-term problem that can be resolved, or at least managed, with other effective evidence-based treatments.
Acknowledgements
None.
Conflicts of interest
None declared.
References
[1] Olson L. Hypnotic hazards: adverse effects of zolpidem and other z-drugs. Aust Prescr. 2008;31(6):146-9.
[2] Dobbin M. Pharmaceutical drug misuse in Australia. Aust Prescr. 2014;37(3):79-81.
[3] Australian Drug Foundation. Prevention research quarterly: current evidence evaluated pharmaceuticals. Melbourne: Australian Drug Foundation; 2008.
[4] Therapeutic Goods Administration. Scheduling basics [Internet]. 2011 [cited 2016 Apr 16]. Available from: https://www.tga.gov.au/scheduling-basics.
[5] Royal Australian College of General Practitioners. Prescribing drugs of dependence in general practice, part A – clinical governance framework. Melbourne: Royal Australian College of General Practitioners; 2015.
[6] Isbister GK, O’Regan L, Sibbritt D, Whyte IM. Alprazolam is relatively more toxic than other benzodiazepines in overdose. Br J Clin Pharmacol. Jul 2004;58(1):88-95.
[7] Brett J, Murnion B. Management of benzodiazepine misuse and dependence. Aust Prescr. 2015;38(5):152-5.
[8] Roxburgh A, Hall WD, Burns L, Pilgrim J, Saar E, Nielsen S, et al. Trends and characteristics of accidental and intentional codeine overdose deaths in Australia. Med J Aust. 2015;203(7):299.
[9] Smith P, Ozturk S. Codeine rescheduling like ‘stopping a butcher selling meat’’ [Internet]. Australian Doctor. 2015 [cited 2016 Apr 19]. Available from: http://www.australiandoctor.com.au/news/latest-news/codeine-rescheduling-akin-to-stopping-a-butcher-s.
[10] Nielsen S, Bruno R, Lintzeris N, Fischer J, Carruthers S, Stoove M. Pharmaceutical opioid analgesic and heroin dependence: how do treatment-seeking clients differ in Australia? Drug Alcohol Rev. 2011;30(3):291-9.
[11] American Psychiatric Association. Diagnostic and statistical manual of mental health disorders: DSM-5. Washington DC: American Psychiatric Publishing; 2013.
[12] World Health Organization. Lexicon of alcohol and drug terms published by the World Health Organization [Internet]. 2015 [cited 2016 May 1]. Available from: http://www.who.int/substance_abuse/terminology/who_lexicon/en/
[13] World Health Organisation. 19th WHO model list of essential medicines [Internet]. 2015 [cited 2016 May 1]. Available from: http://www.who.int/medicines/publications/essentialmedicines/EML2015_8-May-15.pdf
[14] Blanch B, Pearson S, Haber PS. An overview of the patterns of prescription opioid use, costs and related harms in Australia. Br J Clin Pharmacol. 2014;78(5):1159-66.
[15] Harrison CM, Charles J, Henderson J, Britt H. Opioid prescribing in Australian general practice. Med J Aust. 2012; 196(6): 380-1.
[16] Royal Australian College of General Practitioners. Prescribing drugs of dependence in general practice, part B – benzodiazepines. Melbourne: The Royal Australian College of General Practitioners; 2015.
[17] Australian Institute of Health and Welfare. National drug strategy household survey detailed report: 2013. Canberra: Australian institute of Health and Welfare; 2013.
[18] Juurlink DN, Dhalla IA. Dependence and addiction during chronic opioid therapy. J Med Toxicol. 2012;8(4):393-9.
[19] Hunter Integrated Pain Service. Reconsidering opioid therapy: a Hunter New England perspective. Hunter, NSW: NSW Government Health; 2014.
[20] Dunn KM, Saunders KW, Rutter CM, Banta-Green CJ, Merrill JO, Sullivan MD, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152(2):85-92.
[21] Guilleminault C, Cao M, Yue HJ, Chawla P. Obstructive sleep apnea and chronic opioid use. 2010;188(6):459-68.
[22] Katz N, Mazer NA. The impact of opioids on the endocrine system. Clin J Pain. 2009;25(2):170-5.
[23] Sacerdote P. Opioid-induced immunosuppression. Curr Opin Support Palliat Care. 2008;2(1):14-8.
[24] Lee M, Silverman SM, Hansen H, Patel VB, Manchikanti L. A comprehensive review of opioid-induced hyperalgesia. Pain Physician. 2011;14(2):145-61.
[25] Dutch MJ. Nurofen Plus misuse: an emerging cause of perforated gastric ulcer. Med J Aust. 2008;188(1):56-7.
[26] Chetty R, Baoku Y, Mildner R, Banerjee A, Vallance D, Haddon A, et al. Severe hypokalaemia and weakness due to Nurofen misuse. Ann Clin Biochem. 2003;40(4):422-3.
[27] Dyer BT, Martin JL, Mitchell JL, Sauven NC, Gazzard B. Hypokalaemia in ibuprofen and codeine phosphate abuse. Int J Clin Pract. 2004;58(11):1061-2.
[28] Lambert AP, Close C. Life-threatening hypokalaemia from abuse of Nurofen Plus. J R Soc Med. 2005;98(1):21.
[29] Feeney GF, Fairweather P. Groin tissue necrosis requiring skin graft following parenteral abuse of buprenorphine tablets. Drug Alcohol Rev. 2003;22(3):359-61.
[30] Feeney GF, Gibbs HH. Digit loss following misuse of temazepam. Med J Aust. 2002;176(8):380.
[31] Taylor D, Kerwin R, Paton C. The Maudsley 2005-2006 prescribing guidelines. London: Taylor & Francis; 2005.
[32] Denis C, Fatseas M, Lavie E, Auriacombe M. Pharmacological interventions for benzodiazepine mono-dependence management in outpatient settings. Cochrane Database Syst Rev. 2006(3):Cd005194.
[33] Lingford-Hughes AR, Welch S, Nutt DJ. Evidence-based guidelines for the pharmacological management of substance misuse, addiction and comorbidity: recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2004;18(3):293-335.
[34] Zitman FG, Couvee JE. Chronic benzodiazepine use in general practice patients with depression: an evaluation of controlled treatment and taper-off: report on behalf of the Dutch Chronic Benzodiazepine Working Group. Br J Psychiatry. 2001;178:317-24.
[35] Ashton H. The diagnosis and management of benzodiazepine dependence. Curr Opin Psychiatry. 2005;18(3):249-55.
[36] Tiller J. The management of insomnia: an update. Aust Prescr. 2003;26(4):78-81.
[37] Barker MJ, Greenwood KM, Jackson M, Crowe SF. Cognitive effects of long-term benzodiazepine use: a meta-analysis. CNS drugs. 2004;18(1):37-48.
[38] Billioti de Gage S, Begaud B, Bazin F, Verdoux H, Dartigues JF, Pérès K et al. Benzodiazepine use and risk of dementia: prospective population based study. BMJ. 2012;345:e6231.
[39] Charlson F, Degenhardt L, McLaren J, Hall W, Lynskey M. A systematic review of research examining benzodiazepine-related mortality. Pharmacoepidemiol Drug Saf. 2009;18(2):93-103.
[40] Sehgal N, Manchikanti L, Smith HS. Prescription opioid abuse in chronic pain: a review of opioid abuse predictors and strategies to curb opioid abuse. Pain Physician. 2012;15(3 Suppl):Es67-92.
[41] Fry C SB, Bruno R, O’Keefe B, Miller P. Benzodiazepine and pharmaceutical opioid misuse and their relationship to crime. An examination of illicit prescription drug markets in Melbourne, Hobart and Darwin [Internet]. 2007 [cited 2016 Apr 23]. Available from: http://www.ndlerf.gov.au/publications/monographs/monograph-21
[42] Kelly E, Darke S, Ross J. A review of drug use and driving: epidemiology, impairment, risk factors and risk perceptions. Drug Alcohol Rev. 2004;23(3):319-44.
[43] Dassanayake T, Michie P, Carter G, Jones A. Effects of benzodiazepines, antidepressants and opioids on driving: a systematic review and meta-analysis of epidemiological and experimental evidence. Drug Saf. 2011;34(2):125-56.
[44] Dwyer J. Coroners Court of Victoria. Coroners Prevention Unit. Drug overdose deaths in Inner North West Melbourne. Presentation to Yarra Drug Health Forum on pharmaceutical misuse. 2013.
[45] Cogger S, Dietze P, Lloyd B. Victorian Drug Trends 2012. Findings form the Illicit Drug Reporting System (IDRS). Australian Drug Trends Series No. 94. Sydney: National Drug and Alcohol Research Centre. University of New South Wales. 2013.
[46] Rintoul AC, Dobbin MDH, Drummer OH, Ozanne-Smith J. Increasing deaths involving oxycodone, Victoria, Australia, 2000-09. Inj Prev. 2010;17:254-9.
[47] Coroners Court of Victoria. Coroners prevention unit – pharmaceutical drugs in fatal overdose: a coroner’s perspective. Melbourne: Coroners Court of Victoria. 2015.
[48] Mitchell MD, Gehrman P, Perlis M, Umscheid CA. Comparative effectiveness of cognitive behavioral therapy for insomnia: a systematic review. BMC Fam Pract. 2012;13:40.
[49] Butler AC, Chapman JE, Forman EM, Beck AT. The empirical status of cognitive-behavioral therapy: a review of meta-analyses. Clin Psychol Rev. 2006;26(1):17-31.
[50] Henschke N, Ostelo RW, van Tulder MW, Vlaeyen MW, Morley S, Assendelft WJ, et al. Behavioural treatment for chronic low-back pain. Cochrane Database Syst Rev. 2010(7):Cd002014.
[51] Macintyre PE, Scott DA, Visser EJ, Walker SM. Acute pain management: scientific evidence. 3rd Edition. Melbourne: Austraian and New Zealand College of Anaesthetists and the Faculty of Pain Medicine; 2010.
[52] Colson J, Koyyalagunta D, Falco FJ, Manchikanti L. A systematic review of observational studies on the effectiveness of opioid therapy for cancer pain. Pain Physician. 2011;14(2):E85-102.
[53] National Institute for Health and Care Excellence. NICE guideline – opioids in paliative care: safe and effective prescribing of strong opioids for pain in palliative care of adults. Cardiff: National Collaborating Centre for Cancer (UK); 2012.
[54] Amato L, Davoli M, Perucci CA, Ferri M, Faggiano F, Mattick RP. An overview of systematic reviews of the effectiveness of opiate maintenance therapies: available evidence to inform clinical practice and research. J Subst Abuse Treat. 2005;28(4):321-9.
[55] Manchikanti L, Ailinani H, Koyyalagunta D, Datta S, Singh V, Eriator I, et al. A systematic review of randomized trials of long-term opioid management for chronic non-cancer pain. Pain Physician. 2011;14(2):91-121.
[56] Ballantyne JC, Shin NS. Efficacy of opioids for chronic pain: a review of the evidence. Clin J Pain. 2008;24(6):469-78.
[57] Noble M, Treadwell JR, Tregear SJ, Coates VH, Wiffen PJ, Akafomo C, et al. Long-term opioid management for chronic noncancer pain. Cochrane Database of Syst 2010(1):Cd006605.
[58] Chen L, Vo T, Seefeld L, Malaric C, Houghton M, Ahmed S, et al. Lack of correlation between opioid dose adjustment and pain score change in a group of chronic pain patients. J Pain. 2013;14(4):384-92.
[59] Schascighini L, Toma V, Dober-Spielmann S, Sprott H. Multidisciplinary treatment for chronic pain: a systematic review of interventions and outcomes. 2008;47(5):670-8.
[60] Ehde DM, Dillworth TM, Turner JA. Cognitive-behavioural therapy for individuals with chronic pain. Am Psychol. 2014;69(2):153-66.
[61] Williams AC, Eccleston C, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev. 2012(11):CD007407.
[62] Wilson SJ, Nutt DJ, Alford C, Argyropoulos SV, Baldwin DS, Bateson AN, et al. British Association for Psychopharmacology consensus statement on evidence-based treatment of insomnia, parasomnias and circadian rhythm disorders. J Psychopharmacol. 2010;24(11):1577-601.
[63] Bandelow B, Sher L, Bunevicius R, Hollander E, Kasper S, Zohar J, et al. Guidelines for the pharmacological treatment of anxiety disorders, obsessive-compulsive disorder and posttraumatic stress disorder in primary care. Int J Psychiatry Clin Pract. 2012;16(2):77-84.
[64] Baldwin DS, Aitchison K, Bateson A, Curran HV, Davies S, Leonard B, et al. Benzodiazepines: risks and benefits. A reconsideration. J Psychopharmacol. 2013;27(11):967-71.
[65] Bond A, Lader M. Anxiolytics and sedatives. In: Verster JC, Brady K, Galanter M, Conrod P, editors. Drug abuse and addiction in medical illness causes, consequences and treatment. New York: Springer; 2012.
[66] Tvete IF, Bjorner T, Aursnes IA, Skomedal T. A 3-year survey quantifying the risk of dose escalation of benzodiazepines and congeners to identify risk factors to aid doctors to more rationale prescribing. BMJ open. 2013;3(10):e003296.
[67] Pallesen S, Nordhus IH, Kvale G, Nielsen GH, Havik OE, Johnsen BH, et al. Behavioral treatment of insomnia in older adults: an open clinical trial comparing two interventions. Behav Res Ther. 2003;41(1):31-48.
[68] Trauer JM, Qian MY, Doyle JS, Rajaratnam SM, Cunnington D. Cognitive behavioural therapy for chronic insomnia: A systematic review and meta-analysis. Ann Intern Med. 2015;163(3):191-204.
[69] Wilson SJ, Nutt DJ, Alford C, Argyropoulos SV, Baldwin DS, Bateson AN, et al. British association for psychopharmacology consensus statement on evidence-based treatment of insomnia, parasomnias and circadian rhythym disorders. J Psychopharmacol. 2010;24(11):1577-600.
[70] Morin CM, Bootzin RR, Buysse DJ, Edinger JD, Espie CA, Lichstein KL. Psychological and behavioural treatment of insomnia: update of the recent evidence (1998-2004). Sleep. 2006;29(11):1398-414.
[71] Hofmann SG, Smits JA. Cognitive-behavioural therapy for adult anxiety disorders: A meta-analysis of randomised placebo-controlled trials. J Clin Psychiatry. 2008;69(4):621-32.
[72] Bystritsky A, Khalsa SS, Cameron ME, Schiffman J. Current diagnosis and treatment of anxiety disorders. Phys Ther. 2013;38(1):30-57.
[73] Sirdifield C, Anthierens S, Creupelandt H, Chipchase SY, Christiaens T, Siriwardena AN. General practitioners’ experiences and perceptions of benzodiazepine prescribing: systematic review and meta-synthesis. BMC Family Pract. 2013;14:191.
[74] Carlsen B, Norheim OF. “Saying no is no easy matter” a qualitative study of competing concerns in rationing decisions in general practice. BMC Health Serv Res. 2005;5:70.
[75] Walter A, Chew-Graham C, Harrison S. Negotiating refusal in primary care consultations: a qualitative study. Fam Pract. 2012;29(4):488-96.
[76] Rogers A, Pilgrim D, Brennan S, Sulaiman I, Watson G, Chew-Graham C. Prescribing benzodiazepines in general practice: a new view of an old problem. 2007;11(2):181-98.
[77] Dyas JV, Apekey TA, Tilling M, Orner R, Middleton H, Siriwardena AN. Patients’ and clinicians’ experiences of consultations in primary care for sleep problems and insomnia: a focus group study. Br J Gen Pract. 2010;60(574):e180-200.
[78] Grimshaw JM, Russell IT. Effect of clinical guidelines on medical practice: a systematic review of rigorous evaluations. 1993;342(8883):1317-22.
[79] Thomas L, Cullum N, McColl E, Rousseau N, Soutter J, Steen N. Guidelines in professions allied to medicine. Cochrane Database Syst Rev. 2000(2):Cd000349.
[80] Armstrong D, Fry J, Armstrong P. General practitioners’ views of clinical guidelines for the management of asthma. Int J Qual Health Care. 1994;6(2):199-202.
[81] Farquhar CM, Kofa EW, Slutsky JR. Clinicians’ attitudes to clinical practice guidelines: a systematic review. Med J Aust. 2002;177(9):502-6.
[82] Lugtenberg M, Burgers JS, Besters CF, Han D, Westert GP. Perceived barriers to guideline adherence: a survey among general practitioners. BMC Fam Pract. 2011;12:98.
[83] Cadieux G, Tamblyn R, Dauphinee D, Libman M. Predictors of inappropriate antibiotic prescribing among primary care physicians. 2007;177(8):877-83.
[84] Blashki G, Richards JC, Ryan P, Pierce D, McCabe MP, Morgan H, et al. Cognitive behavioural strategies for general practice. Aust Fam Physician. 2003;32(11):910-7.
[85] Blashki G, Morgan H, Hickie IB, Sumich H, Davenport TA. Structured problem solving in general practice. 3. Aust Fam Physician. 2003;32(10):836-42.
[86] Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict. 1989;84(11):1353-7.
[87] Wesson DR, Ling W. The Clinical Opiate Withdrawal Scale (COWS). Psychoactive 2003;35(2):253-9.
[88] Busto UE, Sykora K, Sellers EM. A clinical scale to assess benzodiazepine withdrawal. J Clin Psychopharmacol. 1989;9(6):412-6.
[89] Webster LR, Webster RM. Predicting aberrant behaviors in opioid-treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6(6):432-42.
[90] NPS MedicineWise. MedicineWise Conditions – key points for managing chronic pain [Internet]. 2015 Mar [cited 2016 May 4]. Available from: http://www.nps.org.au/conditions/nervous-system-problems/pain/for-individuals/pain-conditions/chronic-pain/for-health-professionals/key-points.
[91] Majumdar SR, Soumerai SB. Why most interventions to improve physician prescribing do not seem to work. Can Med Assoc J. 2003;169(1):30-1.