Reflection of a paediatric cardiac surgery elective demonstrating high-level care. Unravelling the broad systems at play that influence clinical outcomes through the lens of history, innovation, and present-day communication strategies.


Reflection of a paediatric cardiac surgery elective demonstrating high-level care. Unravelling the broad systems at play that influence clinical outcomes through the lens of history, innovation, and present-day communication strategies.

Improved collaboration between doctors and veterinarians is needed to tackle challenges facing human and animal health. This article discusses the history and importance of One Health.

A vast array of medical conditions affects the central nervous system (CNS), implying a tremendous scope of therapeutic interventions that must target the brain. However, all medical therapy to the brain faces the inherent physiological obstacle of the blood-brain barrier (BBB). Furthermore, after the BBB, drugs must navigate the additional barrier of the brain extracellular space (ECS), which presents its own unique biochemical obstacles. Both the BBB and brain ECS present considerable difficulties for drug therapy to treat diseases affecting the brain. With advancing technology, there has been significant progress towards the goal of overcoming these barriers. An exciting development is the use of MRI-guided focused ultrasound (MRIgFUS) to deliver drug-loaded nanoparticles (NP).
This article describes and explores the use of MRIgFUS and NPs, together as a novel method in CNS drug therapy. First, the basic scientific principles underlying the approach are described. Then, studies that demonstrate key concepts, advancements, strengths, and limitations are discussed to outline directions that have been pursued towards the goal of implementing MRIgFUS NP delivery in practice.
Introduction:
The World Health Organisation recommends that all pregnant women receive at least four antenatal visits. However, nearly half of all women worldwide, particularly in less developed countries, do not receive this care. Antenatal care (ANC) provides an opportunity to improve the outcome of pregnancy and reduce maternal and fetal mortality rates, particularly in low- and middle-income countries.
Summary:
There is a critical need for evidence-based studies surrounding ANC and its provision and uptake, both in Australia and on an international level. This is to ensure that the care provided is specific to the needs of every woman the medical community serves. In this article, we examine a Cochrane review of a variety of community-based and health systems-related approaches that target determinants of reduced ANC coverage. The review aims to address the issues affecting antenatal care coverage, highlight the gaps in the care we currently provide, and discusses its implications for the current healthcare policies regarding ANC provision. While transport and cost remain the basic barriers to accessing ANC, woman-doctor partnerships, contextual care, women’s satisfaction, and cultural safety are also of paramount importance if ANC is to reach more women. The part clinicians play, particularly in delivering holistic and woman-centered care, must also be realised in order to restructure care to be more coordinated and effective.
Michelle Obama once said, “Communities and countries and ultimately the world are only as strong as the health of their women [1].”
Antenatal care (ANC) plays an important role in assisting and preparing pregnant women mentally, emotionally, and physically for childbirth. ANC ensures that the well-being of both the mother and child is well monitored to ensure an ongoing pregnancy with an increased likelihood of a successful birth and a healthy baby. Despite the importance of ANC for both mother and child, globally only 82% of pregnant women have access to at least one ANC visit during their entire gestation period, and only 54% receive the recommended minimum of at least four antenatal visits [2,3]. It is important to discern and address the causes of this disparity. A 2015 systematic review by Mbuagbaw and colleagues analysed a variety of community-based and health systems-related interventions that targeted determinants of reduced ANC coverage, in order to ascertain their effectiveness in increasing the number of women who received ANC [4]. This article analyses and interprets the findings of the review, exploring its implications for women, clinicians, and the broader medical community.
The review evaluated results from up to 400,000 women across 34 randomised controlled trials assessing different methods of optimising antenatal care. Of these trials, 29 used a cluster-randomised design. The trials tested two main types of interventions both aimed at improving the uptake of ANC: health system interventions that included home visits for pregnant women and provision of adequate equipment for clinics; and community-based interventions, such as media campaigns, provision of education, or financial incentives for pregnant women.
Using one intervention, as opposed to none, was found to be effective as demonstrated by an increase in the number of women who received four or more antenatal visits, an increase in the number of women who received at least one antenatal visit, and the number of births carried out in a health facility. There was no evidence of any change in the number of pregnancy-related deaths or any impact on the rates of low birth weight babies [4].
Using a combination of interventions in comparison to no intervention resulted in an increase in the proportion of pregnant women who obtained at least one antenatal visit. Combined interventions also resulted in a reduction in perinatal deaths and a reduction in underweight babies, compared with no intervention. There were no differences between single interventions and combined interventions for any outcome measured [4].
The findings revealed that regardless of the number of interventions used the implementation of at least one intervention led to a positive outcome. In view of this, it is important to evaluate the methods used and to understand and appreciate why the interventions were successful.
Interpretation of the findings
Two main categories of interventions were used in the systematic review, as detailed above. The first targeted the health system and involved the reorganisation of health services and patient-centred care. This proved effective as it addressed the woman’s sociocultural context and agenda, serving as an example of patient-centred care as defined by the Institute of Medicine [5]. Both personal and social environments play a role in influencing the experience of pregnancy. It is therefore essential to consider these factors when administrating health services to ensure that the best care is provided and the needs and expectations of the woman are met [6].
The majority of the trials were implemented in low and middle income countries. Most pregnant women in these populations are still unable to gain access to healthcare and thus experience poorer health outcomes [7]. In a similar study that evaluated the factors affecting ANC attendance across four sub-Saharan regions, the results showed that the way women described ANC was often vague: many of the women had very generalised descriptions about care during pregnancy, what it comprised of, what transpired when one was administered ANC, and the necessity of at least four visits [8].
Moreover, the women often only sought ANC when they faced problems or uncertainties with their pregnancies. How the ANC services responded to these uncertainties, together with their general interactions with the pregnant women, affected women’s ANC attendance [8].The attitudes and behaviours of healthcare workers has long been recognised to influence patient care. Poor interpersonal relationships may act as a barrier to the successful conveyance and interpretation of information, a key component of a successful patient-centred interview [9].
In view of these results, the second intervention that targeted the community proved to be highly effective. The methods used were aimed at helping women, particularly pregnant women, gain a greater understanding of the purpose of ANC. A study carried out in Pakistan’s Punjab province showed that women’s lack of awareness of ANC was also responsible for low ANC coverage [10]. With limited knowledge, the use of ANC services is reduced. As health professionals, it is important that we never assume a woman has any previous health knowledge. Provision of information regarding the services rendered and their usefulness can prompt more women to use the available services while ideally improving patient satisfaction [11]. In addition, the provision of education about health in pregnancy should be culturally appropriate, including supplying a local adaptation of the written materials, making them culturally and linguistically applicable to the target population [6].
The second intervention additionally offered financial incentives, which increased access to ANC for women who were previously deterred due to its cost. It also addressed social mobilisation, which consequentially actuated community initiatives and creativity in addressing the problem at hand. This intervention also included changes in behaviour, such as birth preparedness, aimed at modifying behaviour patterns that can cause low ANC uptake.
Implication of the systematic review findings
A woman-centred healthcare system involves the meaningful engagement of women and the formation of partnerships with the woman and their families. The trust that arises as a result of a strengthened woman-provider relationships has the power to drive change in healthcare delivery. Taking time to build rapport helps improve women’s experience of ANC. It requires effective communication as women who understand their healthcare providers are more likely to understand their treatment and adhere to follow-up recommendations [12].
“Put patients first” declared Harvey Fineberg, President of the Institute of Medicine. “When one has truly understood what the patient needs, they have truly put the patient first [13].” Both interventions used in this study involved reaching out to individual women. Findings revealed that the usage of ANC was considerably lower in women who lived far from the place ANC was delivered, as the long distances reduced access. This was largely the case for women living in rural and remote areas [14]. The use of mobile clinics and greater involvement of the healthcare system, such as requiring skilled attendants to make home visits to pregnant women in remote communities, would greatly reduce such problems. Mobile clinics in particular, as an integral part of the healthcare system, have proven to be highly beneficial in the provision of high-quality, low-cost care to vulnerable isolated populations. They offer a wide scope of services tailored to the community’s needs, thus removing the logistical constraints (transport and financial issues, long waiting periods, and complex and often tedious administrative practices) faced by many [15].
Adequate improvements in the utilisation of ANC and thus its coverage require much more than an increase in the health workforce or an increase in the number of health centres established. It demands a greater focus on a woman’s overall social, political, and economic determinants of health. When ANC provision is both theoretically and contextually opposed to local beliefs and experiences, its usage is diminished, especially when women experience any form of abuse in their care setting or when their attendance puts them at risk from their immediate family or community [16].
Implications within an Australian context
In 2002, Hunt published research aimed at improving ANC, its protocols, and practice in the Northern Territory in Australia [17]. He suggested that antenatal visits be prolonged in time, but less in number, thus making them more likely to be comprehensive and delivered in a more flexible woman-centred manner that makes no generalisations or assumptions about its patients. Likewise, the Daruk Aboriginal Community Controlled Medical Service in New South Wales succeeded in achieving earlier ANC attendance and increasing the number of ANC visits through a comprehensive primary healthcare program. This program incorporated a wide range of ANC services, including home visits and transport provision, both examples of strategies which could be extended to many other Aboriginal communities [18,19]. Conversely, a large proportion of Aboriginal and Torres Strait Islander people live in urban or inner regional areas and have their healthcare channelled through mainstream services. Therefore, it is imperative that we optimise the care we provide to these groups by applying the same principles of cultural competence in all healthcare services, in order to heighten the authentic involvement of women in decision-making. Such measures have the potential to see a greater proportion of this population gain access to the services available to them [19].
These interventions could prove to be very useful in many rural and remote regions, and specifically the Aboriginal community through the engagement of the wider community. The assistance from Aboriginal health workers facilitates communication and understanding between the woman and the healthcare provider, which may consequentially engender trust and responsiveness to ANC [20].
Recommendations
Therefore, when providing care as health professionals, we need to consider the woman’s context and establish a holistic approach that addresses the needs and concerns of that specific woman [17]. This is pertinent in places where culture plays a pivotal role. ‘Shame’ in Australian Aboriginal communities is a culturally-held belief that introduces behaviours and attitudes, evident in patient-doctor encounters, that can be easily misconstrued, resented, and/or disregarded by care providers who fail to appreciate its role in a woman’s life and family [18]. A basic yet appreciative understanding of the history and policies that have moulded the lives of Aboriginal women and their families may assist in the comprehension of some of their health behaviours. For example, the systematic removal of Aboriginal children from their families, the Stolen Generation, has been suggested as a prevailing source of distrust in the Aboriginal mothers’ community [19,20]. Provision of a healthcare service that is culturally equipped to provide holistic ANC is essential if we are to successfully reach out to all communities, including the Aboriginal community.
Based on the findings of this systematic review, it is evident that several interventions were effective in increasing ANC coverage and improving other pregnancy-related outcomes. Reported interventions addressed the common problems that affected the utilisation of ANC, which included maternal knowledge, accessibility to healthcare facilities, and financial difficulties. Accordingly, as doctors and future practitioners, it is imperative that as we provide maternal and antenatal care, we structure the healthcare services we provide around the woman and cater to their individual preferences, needs, and concerns. We are advised to accommodate the woman as much as we can, which means providing them with care that is specific to them and care that addresses the whole person [15-17]. The evidence obtained in Mbuagbaw’s review should be applied effectively in all areas, especially in those places with low ANC coverage [4]. This also serves as an indicator of the gaps in the current evidence that still require further research.
Therefore, instead of asking “Why do women not accept the service that we offer?” the important question should be “Why do we not offer a service that women will accept [22]?”
Conflict of interest
None declared.
[1] Obama M. A plea for education [Internet]. TED talks; 2009 [updated 2009 Apr 2; cited 2016 Apr 2]. Available from: http://www.ted.com/talks/michelle_obama?language=en
[2] Only half of women worldwide receive the recommended amount of care during pregnancy. UNICEF Data: Monitoring the Situation of Children and Women [Internet]. The United Nations Children’s Fund; 2015 [updated 2015 Jul; cited 2016 Mar 14]. Available from: http://data.unicef.org/maternal-health/antenatal-care.html
[3] Antenatal care: Global Health Observatory (GHO) data [Internet]. Switzerland: World Health Organization; 2017 [updated 2017; cited 2017 Mar 13]. Available from: http://www.who.int/gho/mdg/maternal_health/antenatal_care_text/en/
[4] Mbuagbaw L, Medley N, Darzi AJ, Richardson M, Habiba Garga K, Ongolo-Zogo P. Health system and community level interventions for improving antenatal care coverage and health outcomes. Cochrane Database Syst Rev. 2015;(12):CD10994. doi:10.1002/14651858.CD010994.pub2
[5] Institute of Medicine, Committee on Quality of Health Care in America: crossing the quality chasm. A new health system for the 21th century. Washington, D.C: National Academy Press; 2001:6
[6] Clinical practice guidelines antenatal care – module I: understanding the women’s context [Internet]. Australian Department of Health; 2013 [updated 2013 Apr 2; cited 2016 Mar 19]. Available from: http://www.health.gov.au/internet/publications/publishing.nsf/Content/clinical-practice-guidelines-ac-mod1~part-a~woman-centred-care~womans-context.
[7] Women’s health: the new national agenda: AWHN position paper [Internet]. Australia: Australian Women’s Health Network; 2008 [cited 2016 Mar 19]. Available from: http://whnsw.asn.au/wp-content/uploads/2016/01/AWHN_Position_Paper.pdf
[8] Pell C, Meñaca A, Were F, Afrah NA, Chatio S, Manda-Taylor, et al. Factors affecting antenatal care attendance: results from qualitative studies in Ghana, Kenya and Malawi. PloS One. 2013;18(1):e53747. doi:10.1371/journal.pone.0053747
[9] Holmes W, Goldstein M. “Being treated like a human being”: attitudes and behaviors of reproductive and maternal health care providers [Internet]. 2012 [cited 2016 Apr 14]. Available from: https://www.burnet.edu.au/system/asset/file/1408/Holmes_et_al_attitudes_review_sept2_final.pdf
[10] Majrooh MA, Hasnain S, Akram J, Siddiqui A, Memon ZA. Coverage and quality of antenatal care provided at primary health care facilities in the ‘Punjab’ province of ‘Pakistan’. PLoS One. 2014; 9(11):e113390. doi:10.1371/journal.pone.0113390
[11] Galle A, Van Parys AS, Roelens K, Keygnaert I. Expectations and satisfaction with antenatal care among pregnant women with a focus on vulnerable groups: a descriptive study in Ghent. BMC Womens Health. 2015;15(1):1-12. doi:10.1186/s12905-015-0266-2
[12] Frampton S, Guastello S, Brady C, Hale M, Horowitz S, Smith SB, Stone S. Patient-centered care improvement guide [Internet]. Picker Institute; 2008 [updated 2008 Oct; cited 2016 Mar 26]. Available from: http://www.patient-centeredcare.org
[13] Cooney E. Put patients first [Internet]. Harvard Medical School; 2013 [updated 2013 May 30; cited 2016 Mar 13]. Available from: http://hms.harvard.edu/news/put-patients-first-5-30-13
[14] Ye Y, Yoshida Y, Harun-Or-Rashid M, Sakamoto J. Factors affecting the utilization of antenatal care services among women in Kham district, Xiengkhouang province, Lao Pdr. Nagoya J Med Sci. 2010;72(1):23- 55.
[15] Hill CF, Powers BW, Jain SH, Bennet J, Vavasis A, Oriol NE. Mobile health clinics in the era of reform. Am J Manag Care. 2014;20(3):261-4.
[16] Finlayson K, Downe S. Why do women not use antenatal services in low- and middle-income countries? A meta-synthesis of qualitative studies. PLoS Med. 2013;10(1):e100373. doi:10.1371/journal.pmed.1001373
[17] Hunt J. How can routine antenatal care protocols and practice in the Northern Territory be improved? A discussion paper [Internet]. Centre for the Study of Mothers’ and Children’s Health, La Trobe University; 2002 [cited 2016 Apr 14]. Available from: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.602.9270&rep=rep1&type=pdf
[18] Kildea S, Kruske S, Barclay L, Tracy S. ‘Closing the Gap’: how maternity services can contribute to reducing poor maternal infant health outcomes for Aboriginal and Torres Strait Islander women. Rural Remote Health. 2010;10(1383):1-18.
[19] Clarke M, Boyle J. Antenatal care for Aboriginal and Torres Strait Islander women. Aust Fam Physician. 2014;43(1):20-4.
[20] Simkhada B, Teijlingen ER, Porter M, Simkhada P. Factors affecting the utilization of antenatal care in developing countries: systematic review of the literature. J Adv Nurs. 2008;61(3):244-60. doi:10.1111/j.1365-2648.2007.04532.x
[21] Stolen Generations History. National Sorry Day Committee [Internet]. National Sorry Day Committee; 2015 [updated 2015 May 24; cited 2016 Apr 12]. Available from: http://www.nsdc.org.au/stolen-generations-history/
[22] Phumaphi J, Evans T, Van Lerberghe WV, Manuel A, Matthews Z, Wolfheim C, et al. Make every mother and child count: World Health Report 2005. WHO: Sexual and reproductive health [Internet]. Switzerland: World Health Organization; 2005 [updated 2005; cited 2016 Apr 2]. Available from: http://www.who.int/whr/2005/whr2005_en.pdf?ua=1

Introduction: Given the prevalence of cancer and its multidisciplinary and increasingly personalised treatments, this group of diseases is an ideal vehicle for teaching medicine and medical care. The teaching of oncology in medical school environments is of paramount importance to the skill sets and efficacy of future doctors. But do we do it well, and could we do it better?
Discussion: Oncology education in medical schools in Australia and abroad is explored through a personal lens, a qualitative survey, and a literature review in this article. First, the author reflects on his own experience of oncology education as a cancer survivor and third-year medical student. Qualitative data from a survey of medical students at one institution provide insight into the potential benefit of increased exposure to people living with cancer while in medical school. Australia’s andragogic oncological landscape is critically evaluated, and opportunities for change are proposed.
As a cancer survivor, the frightening experience of a cancer diagnosis is something I intimately understand. Cancer remains the major cause of mortality in Australia and many cancers still have very high mortality rates [1]. One in two Australians will be diagnosed with a cancer before the age of 85. In 2016, it was estimated that 130,466 people were diagnosed with cancer [2] and 46,880 people died from cancer. In this same year, 384,593 Australians reported living with cancer [2].
 Despite large volumes of research, it is a disease that continues to kill indiscriminately and its treatment inflicts harsh side effects during therapy and late toxicities in survivorship. The contradictory way that the causes of cancer are communicated and the complexity of the disease all contribute to a fear of cancer. Medical students are not immune to this fear and many develop additional anxiety about cancer throughout their university training. Cancer, for medical students, is a complex disease process, often of unfamiliar and unknown aetiology. It challenges our understanding of genetics, anatomy, physiology, therapeutics, psychology, and public health. As a major health burden, all doctors and medical students should possess a foundational understanding of cancer screening, the pathophysiology underpinning its signs and symptoms, principles of diagnosis and cancer treatment, and appreciate the importance of multidisciplinary care. Simultaneously, doctors require the confidence to effectively and empathetically facilitate conversations with patients and their families about the diagnosis of cancer, fear and grief, prognosis, side-effects, palliative care, and end-of-life planning [3-5].
Despite large volumes of research, it is a disease that continues to kill indiscriminately and its treatment inflicts harsh side effects during therapy and late toxicities in survivorship. The contradictory way that the causes of cancer are communicated and the complexity of the disease all contribute to a fear of cancer. Medical students are not immune to this fear and many develop additional anxiety about cancer throughout their university training. Cancer, for medical students, is a complex disease process, often of unfamiliar and unknown aetiology. It challenges our understanding of genetics, anatomy, physiology, therapeutics, psychology, and public health. As a major health burden, all doctors and medical students should possess a foundational understanding of cancer screening, the pathophysiology underpinning its signs and symptoms, principles of diagnosis and cancer treatment, and appreciate the importance of multidisciplinary care. Simultaneously, doctors require the confidence to effectively and empathetically facilitate conversations with patients and their families about the diagnosis of cancer, fear and grief, prognosis, side-effects, palliative care, and end-of-life planning [3-5].
During my first two years of medical school, I was surprised to not once encounter a cancer advocate or patient as part of my learning. This was surprising because patient narratives and engagement are regarded as central to medical education. Early exposure in preclinical years to cancer patients and oncology content has been shown to improve communication confidence and increase empathy towards cancer patients [6].
I am open about my stage-three colorectal cancer journey [7] and it felt natural to share my lived experience with my problem-based learning team of seven other students during first year. Learning about cancer within this context I believe benefited me and my colleagues. Their questions and knowledge allowed me to conceptualise my own experience in new ways, and helped me retain a greater amount of the material encountered during oncology cases. The prevalence of cancer and growing incidence of certain cancers in young adults means medical students will be increasingly confronted by cancer in some way during their learning. This should be embraced by medical curricula and extracurricular structures as a strategy to create more rewarding oncology learning environments that draw on lived experience within medical cohorts (when appropriate) and cancer groups in the wider community.
When in second year, I asked members of my first-year problem-based learning group to reflect on their experience of learning about cancer alongside me. At my medical school, we have traditional lectures, occasional workshops, and frequent tutorials with a problem-based learning group. Its membership remains the same for the academic year. Oncology is not taught as a block, but is weaved throughout the curriculum. There were no specific tutorials or workshops on oncology, but from time to time discussion in my tutorial group would turn to oncology and my experience and we would have involved discussions together.
An eight-item survey was distributed via SurveyMonkey to the seven other members (average age 24 years old; five females and two males) of the learning unit in April 2016 and data was anonymously collected from five respondents (two colleagues did not provide data for reasons I did not ascertain to protect anonymity). Closed questions concerned the quality of oncology teaching (for example: Do you believe the amount of oncology education provided by the school’s current curriculum is ideal? and Would you have liked more contact with cancer survivors last year?) and learning about cancer alongside a cancer survivor (for example: Was your cancer learning experience advantaged, disadvantaged or unaltered by the presence of someone in your problem-based learning group with direct lived experience with cancer?). Two questions asked students to list the topics they believed they would be most comfortable with when entering hospital and which format for learning about oncology was the most appreciated (for example: lectures, workshops, or tutorials). Two open questions asked for opinions on the role of cancer consumers in education and any other reflections related to oncology education.
Overall, students reported that contact with a colleague living with cancer was transformative, broadened their understanding of oncology, and helped form connections between the biological and humanistic aspects of cancer (Table 1). All five respondents indicated that their cancer learning experience had been positively influenced by the presence of a cancer survivor, and four of the five respondents indicated they would have preferred more contact with cancer survivors in their first year of medicine.

This small sample is neither robust nor generalisable, but these qualitative impressions demonstrate that early exposure to cancer survivors in a medical education environment was a transformative experience for these preclinical students. Given the nature of this survey, sources of bias are numerous and include the sample size, familiarity between myself and the subjects, that the benefits are self-reported only, and that the survey was done several months after the problem-based group was dissolved. However, learning alongside me and hearing about my disease informally throughout the year created patterns of change seen in more robust assessments of oncology educational experiences discussed below.
Given the benefits of early patient contact, why is contact with cancer patients not standard practice in medical schools? To address this question, this article evaluates the historical development and current status of oncology teaching more broadly.
The education of medical students must change over time in response to the changing needs of patients, society, and governments. Cancer education for Australian medical students has a history of responding and adapting to external drivers of change. In 1988, Cancer Council Australia released guidelines for core competencies for medical graduates and called for compulsory oncology education in all medical schools [3]. These guidelines were reviewed and amended as the Ideal Oncology Curriculum for Medical Schools in 1999 and in 2007 by the Oncology Education Committee [3]. Over the same period, a call for a foundation oncology curriculum in Europe was made in 1989. The independent International Union Against Cancer released a paper on oncology curricula in 1994 and an Australian Government inquiry into breast cancer in 1995 concluded that medical schools should urgently develop curricula enabling students to better acquire knowledge about the diagnosis and management of cancer [3].
Medical schools have undergone significant changes in the last decade, including growth in graduate-entry medical degrees, problem-based curricula, student-directed learning, and integrated content. Despite the increasing agility of medical schools to adopt improved teaching and learning models for oncology [5,8-10], assessments of medical students’ understanding of oncology have consistently found knowledge and skill deficits [5,10-16]. The curriculum is crowded, medicine grows more complex, and the volume of information available about a given topic is now unmanageable. This is on a background of a general fear of cancer that many students likely possess. The disjunction between students’ knowledge of cancer and the knowledge they are expected to have remains an active and rich research space in Australia and globally.
While the diagnosis of cancer is usually confirmed by oncologists, the responsibility for facilitating screening and detection remains primarily in the domain of general practitioners (GPs). GPs also provide the majority of general health care for cancer-diagnosed patients [17]. The role of the GP in cancer care and long-term follow-up is growing due to increasing cancer incidence and survivorship, the shift towards defining cancer as a chronic disease, and proposed policy changes aimed at restoring GPs as the keystone of chronic disease management [17-19]. Although approximately half of Australian medical graduates go on to train as GPs, access to oncology training is limited outside of specialist oncologist training programs. Thus, effective oncology training during medical school is of paramount importance in establishing an appropriate skill set for internship and beyond. Further, as the numbers of cancer survivors and people living with cancer grow, cancer care and support issues will impact an increasing number of specialists outside of oncology.
The role of medical curricula is to prepare students for their first year after university as interns. However, what students should know by this point will differ depending on the views of various stakeholders. From a patient perspective, medical graduates need to be able to recognise and identify cancer, understand treatments, engage in discussion around the psychosocial implications of cancer, and appreciate the roles of different health professionals involved in cancer journeys. From a senior doctor perspective, medical graduates should understand history-taking and examination, red flag symptoms, screening and diagnosis, treatment modalities and goals, chronic care needs, communication, and ethics [5,20]. From a public health perspective, medical graduates should understand the principles and guidelines for cancer prevention, screening and detection, and the relationships between cancer prevalence, demography, and geography [5,20]. Last but not least, medical graduates have expectations of their own cancer knowledge: they expect to know about cancer prevention, patterns of cancer prevalence, and the signs and symptoms of cancer. They also strive to effectively communicate with cancer patients, and be up-to-date on the principles of surgery, chemotherapy, immunotherapy, and radiotherapy.
Two major surveys of Australian medical graduates’ attitudes and understanding of cancer were conducted in 1990 and 2001 [4,13]. According to these samples, students in 2001 had more exposure to palliative care and radiation oncology and better knowledge about breast cancer over other cancer types [4]. Procedural-based skills such as performing a Papanicolaou smear and analysing a pigmented skin lesion worsened, but breast examination competency and an understanding of screening guidelines for cervical cancer improved [4]. In the eleven years between surveys, the number of students who felt dissatisfied with the teaching of curable versus non-curable cancer management grew, while the number of students reporting little or no skills in discussing death with dying patients fell [4].
Overseas, a survey of medical graduates in the UK in 2005 found that only 61% of students completed a clinical attachment or special module in oncology, three-quarters would have liked more teaching on oncology, namely radiotherapy, chemotherapy, and symptom management, and only 40% felt prepared to care for cancer patients [14]. Evaluation of American medical graduates found that many lacked knowledge on cancer prevention and history-taking, alongside an unpreparedness to care for cancer patients [5]. In Canada, a 2011 survey found that only half of medical schools taught oncology as a separate topic [11]. It also found that 67% of final-year students felt that oncology education was inadequate and the most poorly taught subject at medical school, a sentiment shared by curriculum committees, residents, and training program managers alike [11].
Medical school oncology education has suffered, and in many places continues to suffer, from neglect, fragmentation, a narrow scope, under-resourcing, and inconsistency between curricula and schools [5,21]. Despite acknowledgement almost 30 years ago of the need for common oncology teaching based on shared principles and standardised learning objectives, disparity in oncology education remains the norm [21,22]. Debate persists around the role of oncologists as educators within medical schools, and how much focus there should be on oncology at medical school. The degree to which oncology should be integrated into curricula [22] or taught as blocks of content is also disputed [23]. Despite a lack of congruence amongst medical oncology teaching, methods to improve oncology teaching have emerged from medical schools around the world.
Standardisation of objectives and curricula
Guidelines for cancer education by medical schools have existed in Australia since 1999 [3]. While these are criticised for not presenting specific learning objectives according for each stage of a medical degree [5], the guidelines are an excellent summary of cancer education objectives that are both patient-centred and skills-oriented. Indeed, Australia should be proud of its national oncology education framework for medical school teaching, as this has yet to be achieved in the United Kingdom [20], Canada [11], or the United States [24].
Teaching methods and resources
Active learning techniques such as problem-based and team-based learning [9] and information technologies are now a common feature of many medical schools. A recent assessment of oncology education across 130 medical schools in the United States found widespread use of case-based learning, online resources, and virtual laboratories; lectures continued to be the dominant form of teaching in all schools [12]. This and other studies demonstrate that medical schools are modernising the methods they use to teach oncology, albeit slowly and despite the predominately online learning styles of medical students [25,26]. Whether newer teaching methods result in improved learning outcomes for medical graduates remains under-evaluated. However, examples of high-yield oncology education strategies are growing and include e-learning oncology modules [27], short clinical oncology modules [28], cancer centre-hosted research programs [8], and summer schools [21].
Use of an oncology textbook remains variable across medical schools. In a Canadian survey of the oncology learning needs of final-year medical students, 89% would have preferred a textbook or web book dedicated to oncology learning objectives [11]. A standard oncology textbook is not recommended across Australian medical schools. However, Clinical Oncology for Medical Students, an e-book produced by faculty representatives across Australian and New Zealand medical schools, is recommended by some institutions and accessible for all medical students to download online [29].
While most Australian medical schools have adopted the concept of learning communities [9], Brazil has expanded student-led and team-focused learning and developed a system of academic leagues with implications for oncology education [30]. The oncology leagues are designed to instil broad knowledge and foster leadership, entrepreneurialism, and learning by engaging in research days, outreach, fundraising, and charitable work [30].
Contact with people living with cancer
Contact with cancer patients is defined as a core clinical experience in the Ideal Oncology Curriculum [3]. However, in Australia, medical student exposure to cancer patients declined from 1990 to 2001 [13]. This is concerning because the need for greater engagement with cancer patients consistently emerges as a theme in surveys of medical graduates, and exposure to cancer patients and hospices is correlated with self-assessments of preparedness for internship [14]. Further, from my small sample of fellow students in my problem-based learning group, contact with me while learning about cancer was beneficial and transformative.
Exposure to patients and advocates is essential to medical school education, and this is particularly the case with oncology because of its complexity, interdisciplinary nature, chronicity, and psychosocial impacts. Solid preclinical and clinical exposure to cancer patients directly assists in the acquisition of skills such as communication, and indirectly via contextualisation and personalisation of the disease [6]. While consumer engagement can be formal and didactic, other models like learning leagues provide the freedom to focus on patient-centred activities such as outreach, education, and support [30]. Since many of the skills needed to understand cancer and communicate with people living with cancer are transferable to other diseases and contexts, investing in better oncology teaching would likely yield benefits across the board for our junior doctors.
The growth in cancer incidence, treatment complexity, and survivorship has resulted in a large amount of discussion about the cancer education of medical students. Calls for medical curricula to remain agile to the shifting needs of cancer patients and communities have echoed widespread concern about the knowledge gaps and unpreparedness of medical graduates to examine, communicate with, and manage cancer patients. Early efforts to standardise oncology learning objectives have largely been resolved in Australia, and medical curricula are slowly adopting newer teaching and learning strategies. However, the exposure of medical students to cancer patients remains unsatisfactory in medical schools in Australia and around the world. In addition, data about what medical graduates understand about cancer should be updated nationally. Building on the existing case-based approach and role of narrative in medical education by drawing upon the well-developed networks of cancer consumers is one way of enhancing the learning outcomes of medical students, but it cannot take place in isolation. Any assessment of oncology education needs to occur alongside a larger discussion about curriculum inclusion, streamlining, and factors accounting for underperforming disciplines such as oncology. For example, there does not appear to be a good rationale for, say, the emphasis on cardiovascular diseases and interventions over cancer at medical school, when heart disease and cancer are both leading causes of mortality and morbidity. Future work will need to identify and then explain differences between subject domains for true medical curriculum reform to begin.
Acknowledgements
Thank you to Professor Alexander Heriot, Dr Ben Ticehurst and Annie Miller for constructive feedback on an earlier version of this article, and the reviewers for constructive suggestions during the review process.
Conflict of interest
None declared.
[1] Causes of death, Australia 2015 [Internet]. Australian Bureau of Statistics; 2015 [updated 2017 Sep; cited 2017 Oct 24]. Available from: http://www.abs.gov.au/AUSSTATS/abs@.nsf/Lookup/3303.0Main+Features12015?OpenDocument
[2] All cancers in Australia [Internet] Cancer Australia; 2017 [cited 2017 Oct 24]. Available from: https://canceraustralia.gov.au/affected-cancer/what-cancer/cancer-australia-statistics
[3] Oncology Education Committee. Ideal oncology curriculum for medical schools [Internet]. The Cancer Council Australia, 2007 [cited 2016 Apr 10]. Available from: http://www.cancer.org.au/content/pdf/HealthProfessionals/OncologyEducation/IdealOncologyCurricDEC07-updatedcover.pdf
[4] Barton M, Bell P, Koczwara B. What should doctors know about cancer? Undergraduate medical education from a societal perspective. Lancet. 2006;7:596-603.
[5] DeNunzio NJ, Joseph L, Handal R, Agarwal A, Ahuja D, Hirsch AE. Devising the optimal preclinical oncology curriculum for undergraduate medical students in the United States. J Cancer Educ. 2013;28(2):228-36.
[6] Granek L, Lazarev I, Birenstock-Cohen S, Geffen DB, Riesenberg K, Ariad S. Early exposure to a clinical oncology course during the preclinical second year of medical school. Acad Med. 2015;90(4):454-7.
[7] Bravery B. The other C word [Internet]. 2011 [cited 2017 Oct 24]. Available from: http://benbbrave.blogspot.com.au/2011/01/other-c-word.html
[8] Fernando E, Jusko-Friedman A, Catton P, Nyhof-Young J. Celebrating 10 years of undergraduate medical education: a student-centered evaluation of the Princess Margaret Cancer Centre-Determinants of Community Health year 2 program. J Cancer Educ. 2015;30(2):225-30.
[9] Ferguson KJ WE, Yarbrough DB, Carline JD, Krupat E. Defining and describing medical learning communities: results of a national survey. Acad Med. 2009;84(11):1549-56.
[10] Matkowski R, Szelachowska J, Szewczyk K, Staszek-Szewczyk U, Kornafel J. Improvements in undergraduate oncology education introduced at Polish medical universities between 2004 and 2010 under Poland’s “National program for combating neoplastic diseases”. J Cancer Educ. 2014;29(3):428-33.
[11] Tam VC, Berry S, Hsu T, North S, Neville A, Chan K, et al. Oncology education in Canadian undergraduate and postgraduate medical programs: a survey of educators and learners. Current Oncology. 2014;21(1):e75-88.
[12] Zumberg M, Broudy V, Bengston E, Gitlin S. Preclinical medical student hematology/oncology eductation environment. J Cancer Educ. 2015;30:711-8.
[13] Barton M, Tattersall M, Butow P, Crossing S, Jamrozik K, Jalaludin B, et al. Cancer knowledge and skills of interns in Australia and New Zealand in 2001: comparison with 1990, and between course types. Med J Aust. 2003;178:285-9.
[14] Cave J, Woolf K, Dacre J, Potts H, Jones A. Medical student teaching in the UK: how well are newly qualified doctors prepared for their role caring for patients with cancer in hospital? Br J Cancer. 2007;97:472-8.
[15] Kujan O, Abuderman A, Azzegahiby S, Alenzi FQ, Idrees M. Assessing oral cancer knowledge among Saudi medical undergraduates. J Cancer Educ. 2013;28(4):717-21.
[16] Deng L, Na FF, Wang JW, Meng MB, He HY, Yang JJ, et al. Insufficient screening knowledge in Chinese interns: a survey in ten leading medical schools. Asian Pac J Cancer Prev. 2011;12(10):2801-6.
[17] Rubin G, Berendsen A, Crawford SM, Dommett R, Earle C, Emery J, et al. The expanding role of primary care in cancer control. Lancet Oncol. 2015;16(12):1231-72.
[18] A healthier Medicare for chronically-ill patients [Internet]. Australian Government Department of Health; 2016 [cited 2016 Apr 9]. Available from: https://www.health.gov.au/internet/ministers/publishing.nsf/Content/health-mediarel-yr2016-ley021.htm?OpenDocument&yr=2016&mth=03
[19] Vision for general practice and a sustainable healthcare system [Internet]. Royal Australian College of General Practitioners; 2015 [cited 2016 Apr 10]. Available from: http://www.racgp.org.au/support/advocacy/vision/
[20] Benstead K, Palmieri C, Brewster A, Gilson D, Jenkins P, Booth J. The minimum competences in non-surgical oncology that medical students need to acquire in order to be safe foundation year 1 (f1) doctors: a Delphi survey. Clin Oncol. 2015;27(7):373-9.
[21] Pavlidis N, Vermorken JB, Stahel R, Bernier J, Cervantes A, Audisio R, et al. Oncology for medical students. Cancer Treat Rev. 2007;33(5):419-26.
[22] Hughes-Davies L, Barrett J. Training the oncologists of the future. Clin Oncol. 2011;23(9):565-8.
[23] Agarwal A, Koottappillil B, Shah B, Ahuja D, Hirsch AE. Medical student-reported outcomes of a radiation oncologist-led preclinical course in oncology: a five-year analysis. Int J Radiat Oncol Biol Phys. 2015;92(4):735-9.
[24] DeNunzio NJ, Hirsch AE. The need for a standard, systematic oncology curriculum for us medical schools. Acad Med. 2011;86(8):921.
[25] Fiche M, Lepori D, Guntern D, Jucker-Kupper P, Jeanneret W, Zaman K, et al. Improving breast cancer education: the case of an evolving multidisciplinary module for undergraduate medical students (Lausanne medical school, 1993–2008). J Cancer Educ. 2010;25:101-5.
[26] Hirsch AE, Handal R, Daniels J, Levin-Epstein R, DeNunzio NJ, Dillon J, et al. Quantitatively and qualitatively augmenting medical student knowledge of oncology and radiation oncology: an update on the impact of the oncology education initiative. J Am Coll Radiol. 2012;9(2):115-20.
[27] da Costa Vieira RA, Lopes AH, Sarri AJ, Benedetti ZC, de Oliveira CZ. Oncology e-learning for undergraduate. A prospective randomized controlled trial. J Cancer Educ. 2017;32(2):344-51.
[28] Auret K, Starmer D. Using structured clinical instruction modules (scim) in teaching palliative care to undergraduate medical students. J Cancer Educ. 2008;23:149-55.
[29] Sabesan S, Olver I. Clinical Oncology for Medical Students [Internet]. Sydney: Cancer Council Australia; 2015 [cited 2017 Oct 24]. Available from: http://wiki.cancer.org.au/oncologyformedicalstudents/Clinical_Oncology_for_Medical_Students
[30] Ferreira DAV, Aranha RN, de Souza MHFO. Academic leagues: a Brazilian way to teach about cancer in medical universities. BMC Med Educ. 2015;15:236.

 We walk with our host between the palm trees and brightly painted bungalows, touring the community care centre for elderly and disabled Fijians. The surrounds belie the grim conditions that must be endured by the patients who live here. There is one patient in particular whom our host wants us to meet — a middle-aged man with an intellectual disability and diabetes who has lived at the home for most of his adult life. He greets us warmly and chats with our host in Hindi whilst we take in his condition. His left foot is extensively bandaged, but the skin left visible is swollen and mottled. Our host, who is the chair of our sister-committee based in Fiji, explains that the patient’s foot has been gangrenous and in need of surgical assessment for the past five years, but they have been unable to facilitate this. His gangrenous foot has frequently been infested with maggots, despite the dedicated care of the facility’s nurse. There is just one nurse employed in the forty-bed facility. Due to limited resources, she must wash the patient’s foot barehanded with soap and water. The nurse shows us her supplies: a scanty collection of antibiotics, antihypertensive medications, metformin, and needles and syringes. Recently, she was grateful when our host provided her with latex gloves to wear whilst caring for the patients. The lack of medical resources and the understaffing of care facilities were just some of the issues we encountered during our brief, but enlightening, stay in Fiji. Through Friends4Fiji, a student-led partnership between Monash University and Umanand Prasad School of Medicine (UPSM), our Fijian counterparts, facilitated a week-long placement and education outreach program. We divided our time between undertaking a placement at the local hospital and assisting in the delivery of an anatomy teaching program for Fijian medical students, led by Dr Michelle Lazarus, a senior anatomy lecturer from our university.
We walk with our host between the palm trees and brightly painted bungalows, touring the community care centre for elderly and disabled Fijians. The surrounds belie the grim conditions that must be endured by the patients who live here. There is one patient in particular whom our host wants us to meet — a middle-aged man with an intellectual disability and diabetes who has lived at the home for most of his adult life. He greets us warmly and chats with our host in Hindi whilst we take in his condition. His left foot is extensively bandaged, but the skin left visible is swollen and mottled. Our host, who is the chair of our sister-committee based in Fiji, explains that the patient’s foot has been gangrenous and in need of surgical assessment for the past five years, but they have been unable to facilitate this. His gangrenous foot has frequently been infested with maggots, despite the dedicated care of the facility’s nurse. There is just one nurse employed in the forty-bed facility. Due to limited resources, she must wash the patient’s foot barehanded with soap and water. The nurse shows us her supplies: a scanty collection of antibiotics, antihypertensive medications, metformin, and needles and syringes. Recently, she was grateful when our host provided her with latex gloves to wear whilst caring for the patients. The lack of medical resources and the understaffing of care facilities were just some of the issues we encountered during our brief, but enlightening, stay in Fiji. Through Friends4Fiji, a student-led partnership between Monash University and Umanand Prasad School of Medicine (UPSM), our Fijian counterparts, facilitated a week-long placement and education outreach program. We divided our time between undertaking a placement at the local hospital and assisting in the delivery of an anatomy teaching program for Fijian medical students, led by Dr Michelle Lazarus, a senior anatomy lecturer from our university.
Medical students in Fiji face challenges that we can scarcely imagine in Australia, extending from their academic resources to their expected time commitment. The information provided to students, and, by extension, doctors, is frequently outdated and incomplete, making it difficult to develop evidence-based medical practices [1]. Despite this, the students we worked with through the committee went beyond the required learning in order to develop a solid foundation. With limited resources, they are unable to rely on specialist journals or opinions, hence students are taught to think creatively, a skill they must rely upon in their future practice [1]. In the university we visited, frequent turnover of university administrators and lecturers had led to disruption of the curriculum such that some cohorts missed out on formal teaching of whole body systems in their anatomy course. Students described being reliant on textbooks and plastic models for anatomy teaching. There is no body donor program for dissection classes, and dissections are limited to animal carcasses at the local abattoir. Senior students must meet significant time requirements, with sixth-year students expected to perform weekly ‘on call’ shifts and night shifts, as well as attending wards on the weekends. These requirements may be explained by the inadequate hospital staffing levels, identified by comparing staff-to-population ratios with the projected numbers needed [2]. Having medical students performing intern-level tasks assists in alleviating doctors’ workloads without costing the national health budget further, as 60% of the budget already directly funds the workforce [2]. This shortage of fully qualified doctors has also been identified as impacting on medical training, causing a lack of post-graduate training opportunities, which contributes to emigration following student graduation [2,3]. With the many challenges these students face, it was deeply satisfying to observe their thirst for knowledge that extended beyond the curriculum, and we feel that we met a genuine need with the teaching program that we implemented.
As mentioned, the challenges faced by these students grow substantially when they become doctors, leading to a crisis of emigration among Fijian doctors and a subsequent growing reliance on foreign doctors [2,3]. ‘Push’ factors, which drive young professionals out of Fiji, include heavy workload and lack of promotion prospects and are coupled with ‘pull’ factors, which draw graduates to overseas positions, such as better pay and training opportunities in developed nations [4]. Ultimately, the high numbers of doctors leaving, particularly from the public sector, have been attributed to a complex career decision-making process involving work-related frustrations, with the primary motivation being income [3,5]. This interplay creates diverse problems, from workforce shortages and a lack of specialist training positions for graduates, to a lack of research conducted and published by Fijian authors. Among students we spoke with, intentions for future employment were divided. For those planning to stay in Fiji, students were motivated by a desire to give back to their community, with a student stating, “we study to help people, and Fiji needs A LOT of help,” and another explaining, “it will also be a good opportunity to actually help the people of Fiji get the best medical services from doctors.” Among students considering leaving Fiji, key motivators were gaining experience and learning advanced medicine, with one student stating, “I do tend to look forward to study and work overseas due to their high standard of education and learning programmes.” Whilst many of the students we spoke with had entered medicine with the very intention of giving back to their community by developing much needed skills, one can appreciate the frustrations they face. Every day, when these graduates go to work, they face shortages of essential medications and supplies, which interfere with patient care. There are also shortages in onshore graduate specialist training programs, which are limited to anaesthesia, medicine, obstetrics and gynaecology, paediatrics, and surgery [6]. These graduate options are a fraction of what is offered internationally, and do not meet the demand for specialists in Fijian hospitals, making it difficult for practitioners to receive adequate guidance for patient care [1,3]. The medical students we met in Fiji had diverse career aspirations. One student had greatly enjoyed her rotation at the psychiatric hospital in Suva, and was keen to pursue a career in this field, noting the dire need for more psychiatrists in Fiji. However, due to a lack of training positions, this student was contemplating leaving Fiji to pursue psychiatry training internationally. Despite the competitive nature of entering the postgraduate training programs, the number of specialists accepted into these programs does not meet public need, with only 48.5% actually working in the public sector following completion of specialist training [3]. Though there were many recognised benefits of working in Fiji, including cultural acceptance and religious responsibility, many doctors in Fiji experience significant frustrations with the facilities, bureaucratic processes, and the salaries they receive, factors that the students we spoke with already identify as concerns [3,5].
The lack of basic supplies and difficulties patients faced when accessing critical healthcare in the Fijian facilities were more significant than either of us anticipated. The Fijian public health system relies on a combination of taxpayer funding and external support through a number of United Nations agencies and nations including New Zealand, Australia, and the USA [2]. During our placement at the local hospital it quickly became apparent how underfunded and under-resourced this system is. Every investigation we considered ordering was carefully scrutinised by our supervising doctor, with a much heavier reliance placed on clinical assessment. This in turn impacts medical education, as students and doctors cannot rely on investigations being available and are hence required to think critically and to have a broad knowledge base. The lack of adequate technology to maintain evidence-based practice has also led to pressure from pharmaceutical companies presenting misleading information to doctors, placing further stress on doctors to avoid influence where possible [3,5]. This was particularly evident with medical imaging, which is limited in Fiji due to a paucity of technicians and facilities. Fiji has just three CT scanners [2] and the one located at our hospital was broken for the duration of our placement, and had been for quite some time. This stems from the fact that much of the machinery is donated second-hand from other nations and there is a reliance on this non-functional equipment, which impacts on delivering care to patients [1,2]. On the wards in the hospital, basic items which Australian doctors would consider essential for patient care were scarce. We particularly noticed the lack of access to products for safe hand hygiene practices and personal protective equipment. The lack of access to this basic equipment, combined with insufficient funds for medicines, were identified as primary concerns among the workforce, further contributing to emigration [2,5]. When it comes to ongoing care, shortages of essential medications make it difficult for the doctors to maintain a regular supply for their patients [6]. All medications are monitored monthly and sourced through the government-funded Fiji Pharmaceutical Services, however “stock-outs” are common [2]. The strategies in place for drug regulation are poor, and the quality of imported drugs is a concern to Fijian doctors due to a potential lack of stringent quality testing [1,2]. It was an incredibly steep learning curve to experience such fundamental differences in resources, and has certainly made us much more aware and grateful for what we have readily available in metropolitan Australian hospitals.
Another major difference identified during our stay was the restricted nature of mental health care in Fiji. As one student we spoke with stated, “mental health is mostly ignored in Fiji.” Despite the national emphasis on institutional care for people with ongoing mental illnesses, there is just one dedicated psychiatric hospital in Fiji, located in Suva [7]. This hospital offers generalised psychiatric services, however, like many other areas in Fiji, there are no sub-specialist psychiatric services available [2]. Within the wider community, psychiatric help is limited. At the hospital where we undertook our placement, those suffering acute psychiatric illness or at risk of committing suicide could be cared for in the euphemistically-named ‘Stress Ward’; however there was no psychiatrist or specialised staff available. As few students are able to undertake placement at the specialised mental health hospital, the stress ward and community placements comprise much of their practical experience of mental health. When asked their thoughts about mental health care in Fiji, students identified it as an improving area that still needed more work, with one student explaining, “five to seven years ago, no one bothered about mental illnesses and brushed it aside. So many women had never heard of the term ‘post-partum depression’. Now they get screened regularly. Mental health was an unaddressed issue in the past but we are getting there.” Whilst progress has been made to reduce stigma associated with mental illness through education and awareness by mental health advocacy groups, 42% of Fijians still report that they would be embarrassed to seek medical help for a psychiatric issue [2,8]. This attitude towards mental illness is far better in urban centres than in more remote regions, and level of educational attainment is positively correlated with receptiveness towards people with mental illnesses [7]. However during our visit to a community care facility, we noted residents with schizophrenia who spent most of each day bed-bound. Whilst these patients have access to antipsychotic medication, there is no access to counselling or rehabilitation workers who could help them return to the community. Standardised clinical treatment guidelines and referral protocols are still being developed by the relatively new Mental Health Clinical Services Network, which hopes to make mental health a priority through advocacy and legislation [2]. The Fijian medical students we spoke to were eager to cultivate the positive trend of increased community awareness and knowledge, combating the stigma of mental illness which predominates in Fijian society.
During our stay and discussions with the Fijian students, we learnt that similar to our own university, there was a slight female predominance of students. However, gender roles in Fiji have a clear effect on the academic and medical culture, something we particularly noticed as an all-female teaching team. In Australia, the challenges that women face in medicine are well documented, from sexual harassment to reduced earning potential [9,10]. However, in Fiji, females face even greater social and economic disadvantage, and this perception pervades many aspects of their academic and healthcare systems [2]. The majority of the lecturers and university administrators we met during our time in Fiji were male, and male authors contribute five times more than female authors in research conducted by Fijians [11]. In saying this, the ‘Learning Evidence-based Medicine and Research in Unison’ program developed by Friends4Fiji has seen an equal number of male and female students from UPSM engage with research, and many students, both male and female, spoke of a desire to develop research skills. Within the wider health workforce, 95% of nurses are female, and despite the medical student ratio observed, two thirds of doctors are male [5]. Community health workers, poorly paid basic healthcare workers in rural village areas, are likewise predominantly female [11]. In the specialty field, however, it seems that change is occurring, with almost 40% of graduate specialists being female, and gender having little impact on decisions to resign [3]. Furthermore, the high number of female medical students may represent an exciting potential shift towards a more equitable future for Fijian women. The student response to our teaching by the end of the week was particularly rewarding. In a program of didactic teaching delivered by male academics, female students expressed being inspired to think of themselves as future educators and academics.
Fiji is a nation of two major ethnic groups; ethnic Fijians make up 57% of the population, and Indo-Fijians make up 37% of the population [2]. Something neither of us anticipated was the emphasis placed on race within the health system, where one of the key characteristics identified on each patient profile is the ethnicity of the patient: Fijian, Indo-Fijian, or other. One of the reasons cited to explain this is the differing epidemiological profiles of the two groups, with ethnic Fijians more likely to contract infectious diseases, and Indo-Fijians more likely to have ongoing non-communicable diseases, particularly cardiovascular disease [2]. However, a number of other key differentiating features have been identified within not only the wider population but also the health workforce, and it was clear that racial differentiation was a part of their culture. Within the wider community, mental health outcomes are far worse among Indo-Fijians, with a suicide rate of 24 per 100,000 compared to four per 100,000 per annum for ethnic Fijians [2]. Within the health workforce, there has been significant Indo-Fijian emigration, with much of this being attributed to the political coup in 2000 against the first Indo-Fijian Prime Minister, leading to disillusionment and ongoing concerns among Indo-Fijian doctors [3]. Conversely, among Fijian researchers, Indo-Fijians contributed more than ethnic Fijians (58% versus 40%) [11]. We observed this racial differentiation in all hospital relationships – patient to patient, doctor to patient, and doctor to doctor – as well as in the education setting. It was much more pronounced than it would be in Australia, and again it was something we needed to learn to adjust to during our stay.
Despite the brevity of our stay, we gained a profound insight into healthcare delivery in an under-resourced setting. We were extremely fortunate to have our stay facilitated by a dedicated group of medical students through the Friends4Fiji committee, and we made lasting friendships that have helped to solidify and grow our partnership. It was a challenging, but valuable, experience to be thrust into a position of responsibility that we had not yet encountered as clinical students in Australia. While on placement in Fiji, we were actively making medical decisions in consultation with our supervising doctor. We came to admire the Fijian students who, despite the challenges of their medical education, are thrust into a role of critical responsibility much earlier than in our program. To be able to apply the knowledge we have gained in our studies and also make a difference in patient care was extremely rewarding. Gaining insights into the lives of these students and seeing the issues facing their nation through their eyes was a unique experience. Given all we gained through this experience, it was extremely rewarding to also be able to help fulfil a need for further anatomy teaching, guided by our dedicated and supportive lecturer, Dr Michelle Lazarus. We also hope to engage the students in joint research projects, furthering our knowledge and developing our evidence-based medicine skills together. Our hope is to continue to grow this partnership between our two universities by fostering relationships between medical students and creating opportunities for student exchange.
Conflicts of interest:
None declared.
References
[1] Lowe M. Evidence-based medicine—the view from Fiji. Lancet. 2000;356(9235):1105-7.
[2] Roberts D, Irava D, Tuiketei D, Nadakuitavuki M, Otealagi M, Singh D et. al. The Fiji Islands health system review. Health Syst TransitTransit. 2011;1(1):6-123..
[3] Oman K, Moulds R, Usher K. Specialist training in Fiji: why do graduates migrate, and why do they remain? A qualitative study. Hum Resour Health. 2009;7(9):1-10.
[4] World Health Organisation. The world health report [Internet]. Geneva, Switzerland: World Health Organisation; 2006. 237p. Available from: http://www.who.int/whr/2006/en .
[5] Francis ST, Lee H. Migration and transnationalism. Canberra, Australia: ANU Press; 2009.
[6] Oman K, Moulds R, Usher K. Professional satisfaction and dissatisfaction among Fiji specialist trainees: what are the implications for preventing migration?. Qual Health Res. 2009;19(9):1246-58..
[7] Aghanwa H. Attitude toward and knowledge about mental illness in Fiji Islands. Int J Soc Psychiatry. 2004;50(4):361-75. .
[8] Roberts G, Cruz M, Puamau E. A proposed future for the care, treatment and rehabilitation of mentally ill people in Fiji. Pac Health Dialog. 2007;14(2):107-10..
[9] White G. Sexual harassment during medical training: the perceptions of medical students at a university medical school in Australia. Med Educ. 2008;34(12):980-6..
[10] Cheng T, Scott A, Jeon S, Kalb G, Humphreys J, Joyce C. What factors influence the earnings of general practitioners and medical specialists? Evidence from the ‘Medicine in Australia: balancing employment and life’ survey. Health Econ. 2011;21(11):1300-17..
[11] Cuboni H, Finau S, Wainigolo I, Cuboni G. Fijian participation in health research: analysis of Medline publications 1965-2002. Pac Health Dialog. 2004;11(1):59-78. .

School refusal is not truancy, but both are serious behavioural problems that can have detrimental consequences. Management of school refusal involves ruling out organic causes and assessing contributing factors, such as anxiety and depression. Empirical treatment involves a collaborative approach of cognitive and behavioural therapies involving the child, parents, and school. This article highlights the heterogeneous nature of school refusal, its identification, assessment and management, and the implications for future research.
At first glance, school refusal appears to be a relatively straightforward phenomenon that all youth may experience at some point during their school years. However, youth-motivated school absence is a significant public health problem affecting schools and households around Australia. Nationally, the average attendance rate of state secondary school students is approximately 85%, with the Northern Territory experiencing the lowest rate of attendance at 75% [1]. In Queensland, an estimated 30% of state secondary school students had an attendance rate below 90%, or had missed more than 20 days of school over one school-year [2]. Of the reasons given, ‘unexplained’ absences accounted for more than 25% of the total absences [3]. Due to financial and legal issues associated with chronic absenteeism, health professionals are put under increasing pressure by parents and schools to find a solution to the child’s ‘problem.’ This strain may lead doctors to write generic medical certificates or practice ‘defensive medicine’ in order to avoid professional and legal risks.

School refusal is not a psychiatric diagnosis, but rather a symptom that encompasses a range of possible diagnoses or social problems. There are many terms in the literature that are used to describe the different types of absenteeism. Firstly, child-motivated absenteeism differs from ‘school withdrawal’ in that the latter refers to situations where a family deliberately keeps their child at home for various reasons, such as due to financial reasons or to care for an ill family member [4]. Child-motivated absenteeism is typically categorised into those with school refusal and those displaying truancy (Table 1). ‘School refusal’ is generally thought to encompass difficulties attending or staying in school, and is associated with extreme emotional distress [4–7]. The child stays home with their parents’ knowledge, despite the parents having made a reasonable attempt to encourage the child to attend school. Children exhibiting truancy, in contrast, are more likely to display antisocial tendencies, such as vandalism and theft, rather than emotional distress [4–7]. Truants’ motives for absenteeism include a lack of interest in school-work, unwillingness to conform to the school’s code of behaviour, and an over-riding desire to engage in externalising behaviour, such as disruptive acts or alternative tangible reinforcers on a school day [4–7]. In addition, parents are often unaware of or disinterested in their child’s school absence. The separation of absenteeism into school refusal and truancy has been criticised for the bias shown to children with school refusal who are often perceived sympathetically and judged to be worthy of treatment, while the term ‘truant’ raises punitive connotations and the need for discipline [8,9]. Due to this bias, children labelled truants are under-represented in current literature and it is unclear whether interventions differ between this group and children with school refusal, particularly due to a lack of strong supporting evidence with regards to the effectiveness of common psychological treatments in groups with externalising behaviour, such as truancy [10,11]. Groups inclusive of differing causes of absenteeism should be a future research objective. Until then, since management of school absenteeism is critical for all youth, it is therefore important to be aware of this bias and assess each child individually and thoroughly.
Approximately 1–5% of all school-aged children will demonstrate school refusal behaviour at some point [6,7]. Although it can occur at any age, school refusal is more common between 5–7 years and 12–14 years of age. These age groups correspond to periods of transition to primary and secondary school, respectively [7]. The prevalence of school refusal seems to be unaffected by gender, socio-economics or intelligence [7,12]. One study showed that a high prevalence of adolescents with school refusal and co-morbid depression also experienced learning difficulties, which may have been a causal factor in their school refusal [13]. In a study assessing parental and familial risk factors for school refusal in children, physical punishment by parents, history of organic disease in parents or the child, and positive psychiatric history in a parent or relative were found to be significant [14]. There have been conflicting arguments in the literature regarding the role of family dysfunctions such as conflict, strict parenting or isolation, and school refusal [15,16]. These various aetiological factors emphasise the heterogeneous nature underlying school refusal and the necessity for future studies with larger sample sizes to assist in delineating predisposing risk factors.
The onset of school refusal can occur acutely, such as on the first day of a new school term, or gradually, such as increasing reluctance to attend school until outright school refusal. Non-attendance can occur sporadically, or continually for weeks or months [6]. The emotional distress that often accompanies school refusal can manifest behaviourally, physiologically, and cognitively [6,7]. Behaviours include remaining in bed, refusing to leave the car, crying, or having temper tantrums. Physiological symptoms include abdominal pain, nausea, vomiting, headaches, diarrhoea, sore throat, sweating, and frequent urination. On a cognitive level, children often have irrational fears about school attendance [7]. A case example of school refusal outlining the presentation, investigations, and management is included (Table 2).
Chronic school refusal has a strong association with anxiety-related disorders [17,18]. Common diagnoses include separation anxiety disorder, generalised anxiety disorder, social phobia, specific phobia, and adjustment disorder with anxiety [7,18]. There appears to also be age-related trends in regards to the diagnoses, for example younger children are often assessed to have separation anxiety whereas adolescents tend to be diagnosed with phobias [7]. These phobias are often in relation to social situations where there is an irrational fear of being criticised. People with an anxiety-related disorder often have co-morbid depression; and certainly, there is a high prevalence of school refusal in children with diagnosed or sub-clinical depression [18].
Children with school refusal can also display argumentative and aggressive behaviour when pressure is exerted upon them to attend school. This type of externalising behaviour leads to many of these children being diagnosed with oppositional defiant disorder [7]. It is important to note that in school refusal, this externalising behaviour is not displayed in multiple settings, but primarily contained to the home environment. By definition, conduct disorder-type behaviours, such as social disregard and violence, are not characteristic of school refusal, but more often associated with truancy.

As with any clinical presentation, a thorough history and medical examination must be taken to rule out organic causes. Only reasonable investigations relevant to the presenting physical symptoms should be conducted [19]. Obviously, if a chronic medical condition were to be uncovered, the primary focus of management would be appropriate referral and education [20].
In addition to a detailed history of the presenting physical symptoms, a health practitioner should consider the predisposing, precipitating, and perpetuating issues of the child’s school refusal in terms of individual, family, school, and community factors. Attention should be given to the family function, the reactions and responses of those surrounding the child to their school refusal, and peer relationships in school [19]. Liaising with the school for information about the child’s attendance records and academic progress would be useful to look for trends in absenteeism and potentially undiagnosed learning difficulties. In addition to days missed, it is also important to enquire about tardiness, early departures from school, missed lessons, and time spent out of class. Discussion about the child’s behaviour and social interactions are vital to exclude victimisation from bullying [21].
Standardised behavioural checklists and mental health scales can be given to the child, their parents, and the school teachers to assist in delineating problems and comparing the severity of behaviours at school and home [19]. The School Refusal Assessment Scale-Revised (SRAS-R) is one of the widely accepted checklists used internationally to assess the functional model of school refusal behaviour, such as positive and negative reinforcements [22]. Determining the functional profile of school-refusing behaviour can also assist in identifying underlying psychiatric diagnoses [23]. The SRAS-R has been verified as having good validity, reliability, and utility [5,24]. However, a recent study has highlighted the ambiguity of certain items on the SRAS-R and suggests that some questions should be removed for improved validity and reliability [25]. The use of the scale can be extended across more generalised populations of school absenteeism, including truancy [26].
Finally, health practitioners should be wary of the possibility of an unsafe home situation that the child may be exposed to, as this can contribute to school refusal. For example, a child may feel anxious and hesitant about leaving home if they feel the need to portray a protective role for a parent under domestic violence [20]. Mandatory reporting laws exist in all Australian states and territories for suspected cases of child abuse and neglect. Given the slight differences amongst states regarding child abuse legislation and protocol, it is advisable to check the details applicable to your jurisdiction [27]. Provisions within legislation protect practitioners from liability for reporting confidential information if the report was made in good faith. If a practitioner is uncertain about reporting, they can contact the local child protection unit or a paediatrician for discussion and advice [28].
A quarter of refusals are estimated to remit spontaneously or are dealt with successfully by the family and school without practitioner intervention [5]. However, school refusal has detrimental consequences for the majority of the remaining cases that go undetected and unmanaged. Short-term consequences include poor academic performance, family conflict, and damaged peer relationships. Long-term outcomes include social isolation, employment issues, and increased risk of ongoing internalising mental health problems and developing a psychiatric illness in adulthood, such as panic disorder and agoraphobia [19,29]. On first presentation, risk factors that indicate a poorer prognosis of returning to school consist of severe or long-standing school refusal, being adolescent or of older age, and having a co-morbid psychiatric illness, particularly depression [7,19].
Although there is a strong emphasis on a timely return to school for refusers, few published studies are available that report the long-term implications of return to school. One study concluded that there was enduring improvement in family relationships after the resolution of the child’s school refusal, however, there appeared to be no difference in the long-term social or emotional adjustment [30]. Another retrospective study found long-term improvement in educational and employment outcomes after school refusal treatment, except for those diagnosed with social phobia and learning difficulties; however, the study was limited by small sample size [31]. Further controlled studies investigating the long-term functional impact of managing school refusal need to be conducted.
The primary objective of treatment for youth displaying school refusal is timely return to school. Behavioural therapies and cognitive behaviour therapy (CBT) have been widely studied and accepted as first line treatment where school refusal is associated with the primary diagnosis of an anxiety disorder [32–37]. Unfortunately, studies are limited by high treatment drop-out rates and inconsistent results on follow-up [33,37]. There is also a lack of randomised controlled trials investigating interventions other than CBT variants, such as group therapy or hypnotherapy [32,38,39]. These limitations indicate the need for future research with better-controlled studies and reproducible results, as well as considering other intervention types. More recent studies have found that CBT is most likely to be successful when it is incorporated into a multimodal treatment method involving the child, parents, and school [40,41]. Due to its heterogeneous nature, some cases of school refusal can be complex and persistent, therefore it is recommended that the child be referred to a paediatrician or to child and adolescent mental health services, which include inter-disciplinary teams, for holistic long-term management, which might include medication.
Child treatment plans should be individually tailored, depending on the psychological basis of their school refusal as well as their developmental level [42]. This may initially include relaxation training for those with substantial physiological manifestations of anxiety. By reducing feelings of anxiety, children are better engaged to utilise various CBT strategies. Cognitive therapy can be used to scope the relationship between a child’s emotions and their behaviour, and ultimately facilitate school attendance by problem-solving and modifying maladaptive cognitions [7,43]. Social skills training is beneficial in addressing any deficits in social skills, which may be contributing to the school refusal, as well as managing the social anxiety associated with talking about their absence to peers or teachers [43]. Improving communication between parent and child is also an important component of CBT. Ultimately, graded exposure and planned return to school should be encouraged, involving the child and their teacher with modification of schoolwork and improved in-class support. Regular positive reinforcement should be used to assist progression, and monitoring for relapse is essential.
Parental and family support is crucial for successful return to school. It has been observed that improvement can be made only through positive influence and when the child is convinced that their parents are determined to achieve regular school attendance [44]. Parents should be taught to employ contingency plans and behaviour management strategies, such as ignoring inappropriate behaviour and positively reinforcing appropriate behaviours. Heeding signs of parental psychopathology and initiating anxiety management education may also help parents maintain their composure and focus when facilitating their child’s school attendance [7,43]. This may include arranging for parents to receive mental health services or marital therapy.
The practitioner and parents should also closely liaise with the child’s school in order to enable a smooth return to school. It is important to educate school staff about school refusal behaviour and associated psychological issues, to dispel potential misconceptions about students with school attendance difficulties. School staff are encouraged to accommodate special arrangements, including modifying schoolwork and assessment, and allowing graduated attendance, such as only coming in for favourite lessons or for the first part of the day. Supportive teachers and ‘peer buddies’ are recommended to ensure the child’s experience of school is positive [43]. Complaints of somatic symptoms should be treated tentatively, and unless the child is clearly unwell, they should remain at school.
Pharmacological treatment may be considered adjunct to the cognitive and behavioural therapies, especially when the child has severe underlying anxiety or depressive symptoms, or when they have not responded to the comprehensive psychosocial treatments offered [45]. Based on existing clinical trial studies, there is little satisfactory evidence that supports the effectiveness of commonly prescribed medications. There appears to be some evidence favouring the tricyclic anti-depressant (TCA), imipramine, with concomitant psychosocial therapy as a superior therapy to placebo combined with psychosocial therapy [46–48]. Short-term outcomes consisted of improved school attendance and reduction in anxiety and depressive symptoms [47]. These positive findings were not maintained in a naturalistic one-year follow up study [48]. TCAs can be problematic due to their unpleasant side-effects and toxicity, including cardiovascular complications [49]. Selective serotonin reuptake inhibitors (SSRI), such as fluoxetine, have weaker evidence of efficacy for school-refusing children, showing no significant difference in therapeutic efficacy between combined CBT-and-fluoxetine therapy and CBT alone [50–52]. Despite this, SSRIs tend to be favourable in practice as they are shown to be effective in reducing depressive symptoms in children and adolescents, and are less likely to cause serious adverse effects [52–54]. Children on medication require regular review for response and side-effects.
School refusal is a challenging problem for health practitioners, families, and schools. Early identification and management reduces the risk of detrimental short- and long-term consequences. Management of school refusal can be complicated and arduous, and needs active participation from the child’s parents and school, as well as possibly paediatricians and mental health services. The preferred management of school refusal involves a multimodal treatment approach and CBT. There should be more awareness and understanding about this mental health risk and the potential for early intervention to promote the health and wellbeing of the future generations.
School refusal is a growing topic of interest in the literature. However, the current literature is largely limited by selection of the absentee population, lack of intervention types, poor sample sizes and follow-up, conflicting outcomes, and lack of long-term sequelae after return to school. In order to establish the efficacy of school refusal interventions, future research could benefit from further adequately powered randomised controlled studies with independent reproducible results. There should also be a focus on investigating groups with different causes of absenteeism, such as truancy, as well as management modalities other than CBT and their long-term effects.
Acknowledgements
My thanks to the Mt Gravatt Child and Youth Mental Health Service for their mentoring and inspiration for this review. Thanks to Dr Randal Moldrich (University of Queensland) for a critical review of the manuscript.
Conflicts of interest
None declared.
[1] Australian Curriculum, Assessment and Reporting Authority. National report on schooling in Australia 2012. Table 17, Student attendance rates, government schools, by year level, sex and state and territory, 2012 (per cent); student attendance rates, government schools, by year level and state and territory, 2008–11 (per cent); [cited 2016 March 26]. Available from: http://www.acara.edu.au/verve/_resources/20151210_ANR_2012_Additional_Statistics_w_Part_9.pdf
[2] Department of Education, Training and Employment. Performance insights: school attendance. Queensland: Queensland Government; 2013 [cited 2016 March 8]. Available from: http://education.qld.gov.au/everydaycounts/docs/performance-insights-report.pdf
[3] Department of Education and Training. School attendance: Absences by reason and school [spreadsheet on the internet]. 2015 [cited 2016 March 8]. Available from: http://education.qld.gov.au/schools/statistics/student-attendance.html
[4] Kearney C. School absenteeism and school refusal behavior in youth: a contemporary review. Clin Psychol Rev 2008;28(3):451-71.
[5] Elliott J. Practitioner review: School refusal: issues of conceptualisation, assessment, and treatment. J Child Psychol Psychiatry 1999;40(7):1001-12.
[6] Tonge B, Cooper H, King N, Heyne D. School refusal: description and management. Current Therapeutics 2002;43(3):55-61.
[7] Heyne D, King NJ, Tonge BJ, Cooper H. School refusal: epidemiology and management. Paediatr Drugs 2001;3(10):719-32.
[8] Lyon A, Cotler S. Towards reduced bias and increased utility in the assessment of school refusal behaviour: The case for diverse samples and evaluations of context. Psychol Sch 2007;44(6):551-65.
[9] Brandibas G, Jeunier B, Clanet C, Fouraste R. Truancy, school refusal and anxiety. Sch Psychol Int 2004;25(1):117-26.
[10] Southam-Gerow M, Kendall P. Cognitive-behaviour therapy with youth: advances, challenges and future directions. Clin Psychol Psychother 2000;7(5):343-366.
[11] Maynard B, McCrea K, Pigott T, Kelly M. Indicated truancy interventions for chronic truant students: a Campbell systematic review. Res Soc Work Pract 2013;23(1):5-21.
[12] Hampe E. Miller L, Barrett C, Noble H. Intelligence and school phobia. Y Sch Psychol 1973;11(1):66-70.
[13] Naylor M, Staskowski M, Kenney M, King C. Language disorders and learning disabilities in school-refusing adolescents. J Am Acad Child Adolesc Psychiatry 1994;33(9):1331-7.
[14] Bahali K, Tahiroglu AY, Seydaoglu. Parental psychological symptoms and familial risk factors of children and adolescents who exhibit school refusal. East Asian Arch Psychiatry 2011;21(4):164-9.
[15] Bernstein G, Borchardt C. School refusal: Family constellation and family functioning. J Anxiety Disord 1996;10(1):1-19.
[16] Bernstein G, Garfinkel B. Pedigrees, functioning, and psychopathology in families of school phobic children. Am J Psychiatry 1988;145(1):70-4.
[17] Jones A, Suveg C. Flying under the radar: School reluctance in anxious youth. School Ment Health 2015;7(3):212-23.
[18] Egger H, Costello J, Angold A. School refusal and psychiatric disorders: a community study. J Am Acad Child Adolesc Psychiatry 2003;42(7):797-807.
[19] Sewell J. School refusal. Aust Fam Physician 2008;37(6):406-8.
[20] Katz F, Leith E, Paliokosta E. Fifteen-minute consultation for a child not attending school: a structured approach to school refusal. Arch Dis Child Educ Pract Ed 2016;101(1):21-5.
[21] Havik T, Bru E, Ertesvag S. School factors associated with school refusal- and truancy-related reasons for school non-attendance. Soc Psychol Educ 2015;18(2):221-40.
[22] Kearney C. Identifying the function of school refusal behavior: A revision of the school refusal assessment scale. J Psychopathol Behav Assess 2002;24(4):235-45.
[23] Kearney C, Albano A. The functional profiles of school refusal behaviour: Diagnostic aspects. Behav Modif 2004;28(1):147-61.
[24] Ingles CJ, Gonzalvez-Macia C, Garcia-Fernandez JM, Vicent M, Martinez-Monteagudo MC. Current status of research on school refusal. European Journal of Education and Psychology 2015;8(1):37-52.
[25] Heyne D, Vreeke L, Maric M, Boelens H, Van Widenfelt. Functional assessment of school attendance problems: an adapted version of the school refusal assessment scale-revised. J Emot Behav Disord [Internet]. 2016 [cited 2016 Oct 26]; Available from: http://ebx.sagepub.com/content/early/2016/07/26/1063426616661701.full.pdf+html DOI: 10.1177/1063426616661701
[26] Haight C, Kearney C, Hendron M. Confirmatory analyses of the school refusal assessment scale-revised: replication and extension to a truancy sample. J Psychopathol Behav Assess 2011;33(2):196-204.
[27] Mathews B, Scott D. Mandatory reporting of child abuse and neglect [Internet]. Australia: Australian Institute of Family Studies; 2014 [cited 2016 March 26]. Available from: https://aifs.gov.au/cfca/publications/mandatory-reporting-child-abuse-and-neglect
[28] Bird S. Child abuse: Mandatory reporting requirements. Aust Fam Physician. 2011;40(11):921-6.
[29] Flakierska-Praquin N, Lindstrom M, Gillberg C. School phobia with separation anxiety disorder: a comparative 20- to 29-year follow-up study of 35 school refusers. Compr Psychiatry 1997;38(1):17-22.
[30] Valles E, Oddy M. The influence of a return to school on the long-term adjustment of school refusers. J Adolesc 1984;7(1):35-44.
[31] McShane G, Walter G, Rey J. Functional outcome of adolescents with ‘school refusal.’ Clin Child Psychol Psychiatry 2004;9(1):53-60.
[32] Maynard B, Brendel K, Bulanda J, Heyne D, Thompson A, Pigott T. Psychosocial interventions for school refusal with primary and secondary school students: a systematic review. Syst Rev 2015;11(12).
[33] Beidas R, Crawley S, Mychailyszyn M, Comer J, Kendall P. Cognitive-behavioral treatment of anxious youth with comorbid school refusal: clinical presentation and treatment response. Psychological Topics 2010;19(2):255-71.
[34] King N, Tonge B, Heyne D, Ollendick T. Research on the cognitive-behavioral treatment of school refusal: a review and recommendations. Clin Psychol Rev 2000;20(4):495-507.
[35] Blagg N, Yule W. The behavioural treatment of school refusal: a comparative study. Behav Res Ther 1984;22(2):119-27.
[36] Heyne D, Sauter F, Van Widenfelt B, Vermeiren R, Westenberg P. School refusal and anxiety in adolescence: non-randomised trial of a developmentally sensitive cognitive behavioral therapy. J Anxiety Disord 2011;25(7):870-8.
[37] Maric M, Heyne D, MacKinnon D, Van Widenfelt B, Westernberg P. Cognitive mediation of cognitive-behavioural therapy outcomes for anxiety-based school refusal. Behav Cogn Psychother 2013;41(4):549-64.
[38] Aviv A. Tele-hypnosis in the treatment of adolescent school refusal. Am J Clin Hypn 2006;49(1):31-40.
[39] Sahel R. Group counselling/therapy as a technique to modify the undesirable school behaviour (school phobia) of children at elementary school level in the state of Kuwait [dissertation on the internet]. Bangor, UK: Prifysgol Bangor University; 1989. [cited 2016 Oct 26]. Available from: http://e.bangor.ac.uk/4189/
[40] Reissner V, Jost D, Krahn U, Knollmann M, Weschenfelder A, Neumann A, et al. The treatment of school avoidance in children and adolescents with psychiatric illness. Dtsch Arztebl Int 2015;112(39):655-62.
[41] Oner O, Yurtbasi P, Er A, Basoglu N. The inpatient treatment process for severe school refusal. Klinik Psikofarmakol Bülteni 2014;24(2):176-9.
[42] Bares C. Emerging metacognitive processes during childhood: implications for intervention development with children. Child Adolesc Social Work J 2013;28(4):291-9.
[43] Heyne D, Rollings S. School refusal. Cornwall: Blackwell Publishers Ltd; 2002.
[44] Berg I. Absence from school and mental health. Br J Psychiatry 1992;161(2):154-66.
[45] Tonge B. Pharmacotherapy of school refusal. Behav Change 1998;15(2):98-106.
[46] Layne A, Bernstein G, Egan E, Kushner M. Predictors of treatment response in anxious-depressed adolescents with school refusal. J Am Acad Child Adolesc Psychiatry 2003;42(3):319-26.
[47] Bernstein G, Borchardt C, Perwien A, Crosby P, Kushner M, Thuras P, et al. Imipramine plus cognitive-behavioural therapy in the treatment of school refusal. J Am Acad Child Adolesc Psychiatry 2000;39(3):276-83.
[48] Bernstein G, Hektner J, Borchardt C, McMillan M. Treatment of school refusal: one-year follow-up. J Am Acad Child Adolesc Psychiatry 2001;40(2):206-13.
[49] Geller B, Reising D, Leonard H, Riddle M, Walsh T. Critical review of tricyclic antidepressant use in children and adolescents. J Am Acad Child Adolesc Psychiatry 1999;38(5):513-6.
[50] Tonge T, Melvin G, Dudley A, Clarke K, Gordon M. The augmentation of cognitive behavioural therapy with fluoxetine for the treatment of adolescent school refusal: a randomised controlled trial. Neuropsychiatr Enfance Adolesc 2012;60(5):S297.
[51] Wu X, Liu F, Cai H, Huang L, Li Y, Mo Z, et al. Cognitive behaviour therapy combined fluoxetine treatment superior to cognitive behaviour therapy alone for school refusal. Int J Pharm 2013;9(3):197-203.
[52] Melvin G, Dudley A, Gordon M, Klimkeit, Gullone E, Taffe J, et al. Augmenting cognitive behavior therapy for school refusal with fluoxetine: a randomised controlled trial. Child Psychiatry Hum Dev [Internet]. 2016 [cited 2016 Oct 26]; Available from: https://www.ncbi.nlm.nih.gov/pubmed/27485100 DOI: 10.1007/s10578-016-0675-y
[53] Qin B, Zhang Y, Zhou X, Cheng P, Liu Y, Chen J, et al. Selective serotonin reuptake inhibitors versus tricyclic antidepressants in young patients: a meta-analysis of efficacy and acceptability. Clin Ther 2014;36(7):1087-95.
[54] Korczak D. Use of selective serotonin reuptake inhibitor medications for the treatment of child and adolescent mental illness. Paediatr Child Health (Oxford) 2013;18(9):487-91.

Chronic pain is an anticipated complication of any surgery despite comprehensive treatment modalities to combat it. The development of chronic pain is attributable to a larger variety of inherent risks. Due to both the individual and social costs of chronic, unremitting pain, the value in preventing its development is paramount. Considering the complex pathophysiology of pain, chronic post-surgical pain (CPSP) development requires a multimodal understanding that involves understanding the physiological, psychological, and social circumstances of the patient. Prevention and management of CPSP starts preoperatively, addressing the patient’s risk factors and expectations to anticipate and create a more personalised plan for pain control. Intraoperative measures include local anaesthesia and pharmacological analgesic therapies. postoperatively, a multidisciplinary approach utilising both pharmacological and non-pharmacological strategies can be used. Pharmacological treatments include individualised opioid-based patient controlled analgesia in conjunction with prostaglandin inhibitors, central nervous system pain receptor modulators, and nerve blocks. Non-pharmacological management includes transcutaneous electrical stimulation and acupuncture. A good understanding of how CPSP develops can aid in managing CPSP that can result in better control of chronic pain.
Pain in the perioperative period is a common and anticipated complication of surgery. Pain can be attributed in part to surgical factors such as nerve injury, inflammation, and infection. In addition, chronic pain development is dependent on a larger variety of putative risks that include past medical history of chronic pain and perioperative anxiety (Table 1) [1]. Chronic post-surgical pain (CPSP) is classified by the International Classification of Disease 11, as persisting pain for at least three months after surgery or tissue trauma, with the exclusion of other or pre-existing causes (such as infection and malignancy) [1,2]. The presentation of CPSP is often variable and may occur in relation to deep tissue or skin trauma at the surgical site, referred from viscero-somatic convergence, or related to nerve injury during surgery [3,4].
While epidemiological accounts vary, the populations at risk of developing CPSP are variable depending on the types of surgery and the likelihood of nerve injury. Current literature indicates that CPSP occur in 10-50% of surgical cases. In orthopaedic surgery related CPSP, up to 5% of all surgical patients report severe disabling pain at one year postoperatively, and a further 10% report lesser pain [1,3,4]. In a UK study of 5000 patients considering frequency and cause of chronic pain in the secondary care setting, 22% of outpatients attributed surgery as the major cause of their chronic pain [3,5]. As chronic pain is difficult and costly to manage and has a major impact on quality of life and productivity, the socioeconomic health burden is potentially enormous considering the volume of surgeries performed annually [1,5].
Formerly, conceptualisation of chronic pain was limited to uni-factorial models of biomedical causality. These models sought to explain pain as corresponding directly to bodily damage with severity being a measurement of extent (for example, using a visual analogue scale or numeric pain rating scale), rather than patient interpretation of injury [1,6]. However, the lack of a clear relationship between extent of tissue/nerve damage and pain severity indicates there is a psychological and sociological component to pain, with the additional influences of motivation and secondary gains (family, work, and “the sick role”) [6]. These aspects were heavily enforced by operant learning factors, which develop into “pain behaviours” of avoidance, and cognitive factors based around beliefs and expectations of pain post-surgery [7]. Patients who have a past medical history including emotional or psychiatric stressors, have increased work-related injuries and claims, negative attitudes to treatments, and previous chronic pain diagnosis are at an increased risk of developing chronic pain syndromes [1,8]. To demonstrate this point, a large 2007 prospective surgical cohort study using preoperative psychological questionaries (Item-36 Short Form Health Survey) with postoperative acute pain scores for 625 patients undergoing minor, intermediate, and major surgeries, found that fear of the long-term consequences of surgery predicted increased pain in the six month postoperative follow-up period, independent of the type of procedure and other somatic factors [7].
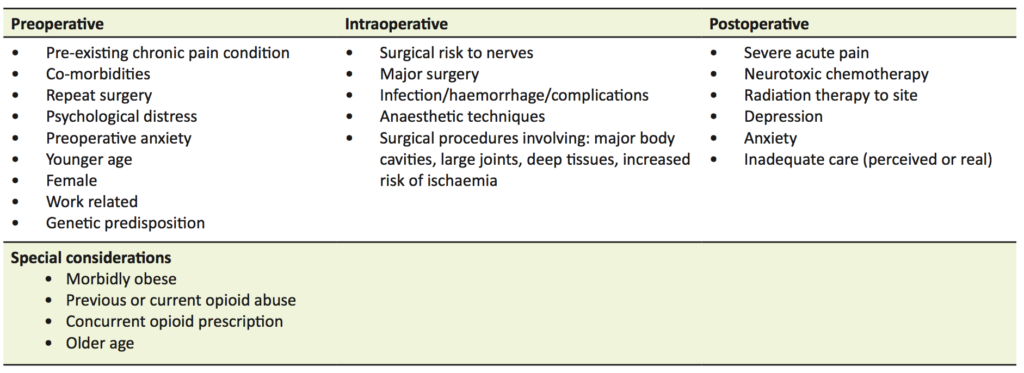
Generally, surgical pain can be attributed to three main mechanisms: inflammation, direct nerve injury, and increased sensitisation [1,2]. Inflammatory pain arising from tissue trauma and ischaemia is an unavoidable aspect of most surgeries [4]. Although the release of local inflammatory mediators like tumour necrosis factor alpha and interleukins 1 and 6 are needed to a certain degree for healing, they can result in hyperalgaesia (augmented sensitivity) and allodynia (misperception of non-painful stimuli) long after the expected healing time from surgery [1,4]. These outcomes are attributed to peripheral nerve sensitisation and are often managed with anti-inflammatory medications both intraoperatively and on an outpatient basis [4]. In conjunction, pain developing from direct nerve injury (compression, stretch, or transection) can have a similar presentation in addition to hyperpathia (exaggerated pain), paraesthesia/dysaesthesia (abnormal sensation) and even hypoesthesia (decreased sensation) [1,4]. For example, nerve injury arising from fetal head descent through the birth canal during labour may result in compression or stretch of lumbosacral nerve roots resulting in radiculopathy from 8% elongation, while 15% elongation can result in axon disruption and axonotmesis [7].
Pain sensitisation is also a major precipitating factor for the transition from acute to chronic pain. The pathophysiology of sensitisation is attributed to increased excitability of both central and peripheral nerve fibres (in addition to decreased inhibition from dorsal horn spinal neurons) [1,10]. Centrally, sensitisation is linked to upregulation of N-methyl-D-aspartate (NMDA) receptors in the dorsal horn causing the “wind-up” phenomenon of pain, with peripheral changes from prolonged inflammation or opioid exposure linked to ‘hyperalgesic priming’ at the afferent sensory nerve level [1,2]. Ectopic activity in transected nerves has also been associated as the underlying cause for the spontaneous pain characteristics of some neuropathic states that involves maladaptive plasticity within the nerve nociceptive system post-injury [1]. It is predominantly owing to this sensitisation (with input from patient psychology) that the major risk factor for developing CPSP is severe acute post-surgical pain, making acute pain management of foremost importance for CPSP prevention [1,2].
Owing to its complexity, pain management warrants a comprehensive and surgery specific multimodal approach [5]. This starts preoperatively with patient risk factors and expectations addressed to anticipate potential complications and acceptable therapies. Furthermore, following procedure specific guidelines produces better clinical outcomes with appropriate discharge and rehabilitation planning incorporating a pain clinic follow-up [2]. For example, open colorectal procedures may benefit from thoracic epidurals to reduce postoperative pain, nausea, and vomiting, while laparoscopic abdominal procedures, with minimal tissue injury, often do not require the same cover [11]. These considerations should be made preoperatively with patient expectations taken into account and the risk of neuropathic/nerve injury considered for procedure appropriate analgesia [2].
Intraoperatively, local and systemic therapies can be used to target the aforementioned biomedical risks and can be further broken into nociceptive and neuropathic targets. Systemic therapies often involve opioid and limited non-opioid options for nociceptive pain, with opioid use being limited by adverse effects (such as respiratory depression and vomiting) [2].
Patient-controlled analgesia (PCA) remains a cornerstone of postoperative pain management, with early postoperative intravenous opioids providing better analgesia than conventional parenteral opioid regimens, with greater patient satisfaction particularly for nociceptive pain [2,11]. There is little evidence that any particular opioid delivered via PCA is superior to another in regard to analgesic or adverse effects in general, but individual patients may tolerate one opioid better than another, and safety of administration can be impacted by hospital staff education [12].
In regards to non-opioid analgesics, there is fair evidence to support their complementary use with opioid analgesics [2]. Medications in this category that target nociceptive pathways include non-steroidal anti-inflammatory drugs (NSAIDs) which are superior to paracetamol (although combining both increases efficacy), and selective COX-2 inhibitors (a subtype of NSAIDs) which offer further advantages over their non-selective counterparts in particular with regard to platelet dysfunction, blood loss, and renal impairment [2,11]. Other multimodal analgesic options that can assist in neuropathic pain management involve intravenous local anaesthetics (such as lignocaine), which has been shown to reduce opioid requirements after abdominal surgery and to decrease the risk of nausea, vomiting, and duration of postoperative ileus, and so decrease the length of hospital stay [12,13]. Locally, lignocaine can also be injected proximally to surgical sites intraoperatively as a preventative somatic analgesia, although a 2005 meta-analysis of 66 randomised controlled trials (comparing preoperative analgesic interventions with similar postoperative analgesic interventions via the same route) and a 2005 randomised controlled trial assessing pain relief in laparoscopic gynaecological surgery suggested the use of pre-emptive local infiltration was associated with a more limited, but still beneficial effect on post-surgical visceral pain [14,15].
The importance of multimodal approaches targeting not only the nociceptive, but also the neuropathic and central neurons can be seen in the prevention of wind-up phenomena and central sensitisation [6,12]. For this, therapies targeting not only opioid, but also substance P, calcitonin gene-related peptide, aspartate, glutamate, gamma-aminobutyric acid (GABA), and NMDA receptors can be used to target pain on multiple levels of the pain pathway [1,2]. Examples of neuropathic treatments include perioperative use of gabapentin and pregabalin, which have both been shown to decrease postoperative opioid requirements [13,16]. Similarly, a meta-analysis of 29 randomised controlled trials indicated a small yet significant decrease in CPSP with intra- and postoperative ketamine use [8]. Furthermore, peri-operative intravenous ketamine also reduces opioid use and postoperative nausea and vomiting compared with placebo, in addition to being cost effective and useful in opioid-tolerant patients [2,17].
Concurrently, the use of regional blocks or neuraxial methods such as femoral nerve blocks may reduce the use and side effects of systemic opioids whilst facilitating early mobilisation and recovery (thereby reducing the psychological impact of illness) [8]. Risk of infection exists, but is predominantly preventable by sterile precautions. Likewise, the risk of neuropathy from this procedure is low and countered generally by the use of ultrasound guidance [11]. Additionally, while regional blocks present an increased risk of procedural complications depending on the block used (for example, brachial plexus blocks risk pneumothorax), these are far rarer than complications of opioid use and often outweigh them on a clinical outcome basis for controlling neuropathic pain [11,18].
In terms of patient education, better pain relief is achieved by structured preoperative education and written information, rather than routine information with generalised verbal discussion [2]. Although identification of preoperative risk factors may assist in targeted patient education and expectation management (Table 1), other interventions, such as pre-surgical hypnosis and music therapy, have been found to be reliable for decreasing CPSP as an outcome indicated by six month follow-up surveys and postoperative pain surveys [1, 7]. Identification of factors that will make pain management more difficult, such as obesity, history of opioid abuse, and current opioid use may assist in appropriate pre and post-surgical management [1,2].
Finally, non-pharmacological methods including transcutaneous electrical stimulation and acupuncture have been shown to reduce postoperative pain, particularly in the setting of back surgery and ambulatory knee surgery when compared to placebo, with the aforementioned psychological methods (distraction, music, and video) being of potential use in paediatric populations. However, the evidence for these is limited and variable in the literature [2,11].
In conclusion, CPSP is a common, inherently complex, and costly complication of surgery. Managing chronic post surgical pain from a multimodal multidisciplinary approach may improve pain control.
References

Mind-body therapies such as mindfulness meditation (MM) are increasingly being studied and applied as legitimate medical therapies. Since becoming popular in the 1970s, MM has been shown to improve psychological states such as anxiety and depression. The scope of MM has expanded in recent years, and MM has been shown to have positive effects on pain, recovery time, and even wound healing after surgery. The number and types of surgery are increasing with the ageing population, and MM has potential as a non-surgical therapy to help hasten recovery, minimise analgesic consumption, and improve overall satisfaction after surgery. Training patients in MM before surgery may be implemented at low cost and up to 24 hours before admission. Given these benefits, complementary mind-body therapies such as MM have potential to improve a patient’s surgical experience and outcomes. Despite the potential benefits, MM is not currently used routinely for patients undergoing surgery. The literature shows that there is a perceived suspicion of the practice’s effectiveness, which appears to hamper its clinical acceptance. Critics cite concerns about patients’ perception of meditation given its religious connotations and whether they would be encouraged to accept MM as a valid therapy. This essay explores the application of MM as a complementary therapy to expedite recovery from surgical admission and concludes that meditation may be as effective as medication in some circumstances.
“The part can never be well unless the whole is well.” This epithet offered by Plato 2300 years ago refers to the symbiotic relationship between mental and physical health, and has increasingly been embraced by Western society [1]. The concept that psychological state can influence physical well-being has contributed to the acceptance and use of mind-body therapies and motivated research into their health benefits. Recent scientific enquiry has noted diverse benefits of meditation such as reduced anxiety and depression levels, improved cardiac health, heightened immunity, and fewer post-chemotherapy adverse symptoms among cancer patients [2-4]. Researchers have also established a strong link between mind-body therapies and pain attenuation [5]. These findings suggest that these therapies may have potential as treatment for elective surgery inpatients.
With the increased number and types of surgical procedures required by an ageing population, meditation has been proposed as a means of improving post-operative outcomes, particularly after elective surgery [6]. Despite reported benefits and potentially low implementation costs [7], traditional medicine has been slow in adopting these alternatives. Critics remain sceptical of the efficacy and practicality of meditation, whereas advocates suggest that the analgesic qualities indicate clinical potential. To reconcile these opposing views, one must consider the logistical, psychosocial, and therapeutic aspects of meditation in the surgical context.
Meditation is often defined as mental exercises and techniques designed to calm the mind through physiological processes [8-10]. Mindfulness meditation (MM) sometimes referred to as ‘Vipassana practice’ or ‘insight meditation’, was thought to have been conceived by Buddhist scholars over 2000 years ago in India and is inextricably linked with Buddhist theology [11]. It involves cultivating a focused psychological attention to the internal and external experiences occurring in the present moment [12,13]. In practice, MM requires attentiveness to simple physical sensations such as breathing, eating, or sitting. Technical applications of this approach vary. One popular methodology in a clinical setting involves using one’s imagination to mentally scan the entire body for awareness of physical sensations without judgment, beginning with the head and progressing to the toes. This can be used for any duration and in many circumstances. MM may also incorporate ‘guided imagery’ techniques in a clinical context, in which the patient visualises his or her own healing process and affirms thoughts of positivity regarding the management of illness [14].
Despite its origins in antiquity, MM has recently been adopted by Western society [15], and today’s incarnation is mostly secular [16]. One of the first occasions of mindfulness being introduced to Western medicine occurred in 1979 by Kabat-Zinn’s Mindfulness Based Stress Reduction (MBSR) program at the Stress Reduction Clinic at the University of Massachusetts Medical Center [3,17]. The inaugural program described reduced self-reported scores for depression and anxiety in participants with psychological problems [11].
To implement MM as a therapeutic tool, Kabat-Zinn adapted the methodology. He anticipated that the introduction of an alternative medicine, particularly one with religious associations, would be denounced by orthodox medical practitioners as the work of charlatans or mystics [17]. Overcoming this prevailing medical stigma was integral to the wider acceptance of mindfulness today. Accordingly, Kabat-Zinn distinguished MBSR from its religious counterpart by exploring the curative potential of meditation and designed it to be used as a clinical tool that complemented rather than replaced conventional medical therapies.
The scope of clinical mindfulness has expanded greatly with wider acceptance of MM by the wider scientific community. Current programs include mindfulness-based cognitive therapy, acceptance and commitment therapy, and mindfulness-based relapse prevention [18,19]. There are now even smart phone applications, DVDs, and self-help books, which have propelled mindfulness concepts into the public domain.
The acceptance of mindfulness by the medical community is also evidenced by the recent interest in the scientific evaluation of mindfulness as a health promotion tool. For example, in the 2008-09 fiscal year, the US government funded hundreds of studies concerning the clinical applications of various meditative practices, at a cost of US $51 million [17].
By influencing psychological states, MM may help address post-surgical complications such as pain and reduced functioning [20]. A systematic review of studies that evaluated psychological variables and surgical outcomes found that psychological state strongly correlates with early recovery, although differences in study design restrict the ability to confidently pool results [20]. Psychological factors have also been shown on occasion to be superior predictors of post-operative outcomes than the surgical intervention itself [14]. Despite continued technological innovation, today many patients endure moderate to severe negative post-operative outcomes [21]. For example, up to 40% of patients who undergo elective joint replacement surgery report suboptimal functional improvement, pain relief, and overall satisfaction after their procedure [22]. These issues suggest that there is a need for complementary therapies to support existing therapies in a surgical setting.
Mind-body therapies such as MM are being increasingly evaluated for their effects on post-operative psychological variables. The use of mind-body therapies as a nonpharmacological adjunct has been well studied in cardiac, abdominal, and orthopaedic surgeries [14]. In these contexts, MM is associated with improved levels of pain, anxiety, fatigue, and distress [14]. Reduced systolic blood pressure has been reported during the post-operative period in patients who have practised a guided-imagery protocol [23]. Other benefits include shorter hospital stay and promotion of wound healing in some studies [14,24].
MM has been shown to be useful for reducing reliance on analgesia in the post-operative period and beyond [14]. Analgesia consumption levels can be used as a proxy for pain control. Although analgesic use is essential for promoting surgical recovery, too great a reliance on pharmaceuticals increases the risk of adverse side effects such as nausea, respiratory depression, and lethargy [25]. Some analgesics can also predispose to long-term dependency if their use is not appropriately stewarded. Palmaro et al. [26] observed that one-third of patients undergoing orthopaedic surgery for carpal tunnel syndrome had persistent and increased consumption of anti-neuropathic and/or opioid analgesics for more than two months after surgery. Among this population, psychiatric disorders and subjective levels of pre-operative pain explained this increased use [26]. MM may positively affect these two variables and reduce medication use. An estimated 234.2 million surgeries are performed worldwide each year, many of these necessitating pain medications [27]. It would therefore make fiscal sense to reduce the amount of pharmaceuticals required after surgery through the use of nonpharmacological therapies such as MM.
Meditative practice has been shown to change brain structure and function [28]. These effects may be seen both immediately and from chronic practice as demonstrated via brain imaging modalities such as fMRI, SPECT and PET [28]. Firstly, the prefrontal cortex (PFC) is intensely active during meditation, specifically the lateral prefrontal regions [28,29]. The ventromedial areas of the PFC are responsible for the affective integration of sensory input, whilst the posterolateral regions are concerned with sensory appraisal without self-referential value [29]. It is proposed that a neuronal shift away from the ventromedial prefrontal regions to the posterolateral centres supports a more self-detached analysis of interoceptive and exteroceptive sensory events [29]. Secondly, additional neural correlates such as modulation of the limbic system contribute to meditative effects [28]. MM practice has been shown to reduce the activity of the amygdala, and broader limbic structures concerned with emotional reactions [28]. For example, after eight weeks of an MM intervention, arterial spin labelling functional MRI showed neuroarchitectural changes such as increasing grey matter concentration within the left hippocampus an amygdala [16]. These regions are associated with emotional regulation, which may account for reduced anxiety and improved coping reported after programs of a similar duration [30]. In addition to this, MM has been posited to exert influence on the hypothalamus, which by extension shifts autonomic nervous system function towards increased parasympathetic activity [28]. This hypothesis attempts to explain physiological reductions in heart rates, blood pressure and serum cortisol levels which all evidence relaxation experienced during MM [28].
Another potential benefit of MM as a surgical therapy is pain modulation. However the exact mechanisms through which MM regulates pain are unknown [3,31]. Zeidan et al. [5] suggested MM can attenuate post-operative pain, reporting a 40% reduction in pain intensity and 57% reduction in pain unpleasantness following mindfulness intervention in a laboratory setting. The authors posited that this phenomenon results from synergistic interactions of improved attentional control, expectation modulation, and a placebo effect. By exerting attentional control on physical sensations other than discomfort, MM is thought to dampen the saliency of nociceptive stimuli.
Although this explanation seems to be reductive at face value, it is consistent with knowledge about complex neurobiology. The influence of MM on neurological pain-modulating networks is only now being explored. The cognitive inhibition of pain has traditionally been attributed to opioidergic mechanisms [32,33]. This model proposes that endogenous opioids are secreted by regions of the brain with an abundance of opioid receptors [33] and that these natural opioids elicit analgesic effects. Opioid receptors are found in high concentrations in the anterior cingulate cortex, orbitofrontal cortex (OFC), and insula [34]. Pain relief attributed to a placebo effect, conditioned pain modulation, and attentional control mechanisms such as those involved in MM rely on opioidergic pain relief [35-37]. These analgesic effects can be reversed after administration with opioid antagonists such as naloxone [34]. Imaging studies have shown that MM-induced analgesia is associated with increased activation in these regions of the brain [34]. This suggests that opioidergic mechanisms may account for some of the analgesic effects associated with MM.
Pain attenuation by MM may be supplemented by non-opioidergic mechanisms because opioidergic and non-opioidergic brain regions work synergistically. In MM, the OFC projects synapses to the thalamic reticular nuclei (TRN) which, via further projections exerts inhibitory control over the thalamus, an area considered to be the ‘gatekeeper’ through which all sensory information must pass [38]. When the TRN is active (either through the OFC or distinct mechanisms) ascending information such as nociception may be filtered from triggering conscious awareness [38]. MM therapy responses might therefore be mediated by the interaction between the OFC and the TRN, which appears to inhibit nociception from reaching the conscious part of the brain, the cerebral cortex. Self-facilitated pain modulatory systems seem to be engaged by non-evaluative recognition of an unpleasant physical sensation such as nociception [38]. Pain reduction experienced during MM is also associated with thalamic deactivation, which suggests a pain-gating effect may be exerted by the limbic system [5]. This suggests that nociception is influenced by the complex interaction of expectations, emotions, and cognitive appraisals, and may be modulated by the meta-cognitive task of focusing on the present moment [5].
MM-based interventions vary in format and administration. Group mindfulness interventions are often preferable in clinical and research settings, and have been shown to expedite improved socialisation, program participation, and skill acquisition [14].
Group therapy with a set number of sessions of prescribed length may be more cost-effective than individual one-to-one interventions [14]. In group formats, a health professional such as a psychologist, physician or nurse instructs participants and distributes supporting material such as books and audiotapes to reinforce the program rationale and encourage independent practice outside standardised sessions.
It may not be practical to offer group sessions for patients undergoing elective surgery because of the nature of elective admissions, which are typically non-emergency procedures and can be delayed or rescheduled at short notice. Patients requiring more urgent surgery would not have sufficient advance notice to begin preoperative group therapy. Therefore, viable program methodologies should be flexible in terms of participant admission or delivered on an individualised basis as part of pre-operative patient care.
Personalised instruction or a single session with a psychologist can be tailored to the patient’s level of comprehension. Patients could also be given the opportunity for follow-up sessions to consolidate skills learned before admission.
Regardless of the mode of delivery, the rationale, advantages, and disadvantages of MM should be explained to the patient before surgery. The patient’s cognitive capacity and psychological state should be assessed by the physician or psychologist to evaluate his or her suitability for MM intervention and provide baseline psychological scores for comparison.
The benefits of regular MM practice in clinical practice have been well documented, and these skills can be consolidated for life [17]. In the context of pre-operative MM programs, optimum duration and timing of MM programs should be considered. The MBSR program developed by Kabat-Zinn [13] spans an 8-week course involving a 20-minute intervention each day. Many clinical programs use a similar program design, which has shown to be adequate to elicit desired benefits [17].
However it does not seem to be necessary for pre-operative programs to be as long as eight weeks to elicit desired effects. MM therapy given for the first time 24 hours before an operation has been shown to be beneficial. For example, Manyande et al. [39] reported reduced scores for post-operative pain and distress, and ward analgesic consumption for surgical patients given a 15-minute audio recording 1 day before elective abdominal surgery. Other studies have reported similar results [18,34,40]. Thus, although the benefits of MM are generally associated with regular practice (which may discourage some from taking up the practice), these findings imply that MM therapy involving short mental training may produce benefits even when undertaken in the days before surgery.
There are potential limitations to MM as a pre- and post-operative therapy for surgical patients. The success of MM programs can be limited by surgery type and patient attributes, such as physical or cognitive impairment [14]. The stress associated with a hospital admission and surgery may impair a patient’s ability to learn a new skill such as MM [41].
Implementation of standardised programs across healthcare providers may require additional funding, development of standardised educational material, and targeted training for healthcare professionals. Estimates of the resources needed would also vary according to differences between practices and institutional infrastructure. However, the cost of implementing MM programs may be recovered at least in part by improved recovery, reduced length of stay, reduced complication rates and reduced analgesic consumption [14,24,39,42]. Further cost-benefit analysis of MM programs for surgical patients may be warranted to better understand the organisational fiscal advantages associated with the use of MM therapies.
The effect size of MM intervention on post-operative outcomes has been subject to debate. Some studies investigating the use of pre-operative mind-body therapies in the surgical context failed to establish changes to post-operative outcomes such as pain and duration of hospital stay [14]. For example, Scott and Clum [43] observed no significant effects of treatments on outcome measures such pain, anxiety and analgesic intake after an attentional control regimen initiated 24hr prior to abdominal surgery. Other studies described mixed outcomes of such protocols [14,39]. It has been suggested that heterogeneity in study design and differences in the surgical context in which they are examined restrict generalizations being formed into the effectiveness of individual protocol design [14]. This is additionally hampered by fluid definitions of ‘mind-body therapies’ and noted methodological flaws consistent throughout much of existing literature, such as reduced sample size, inadequate controls and insufficient study duration [14,17]. Additionally, it is difficult to account for the influence of external factors on broader research outcomes. Factors such as insurance coverage may exert control on measures such as duration of hospital stay which may distort findings [14]. Further research may need to be conducted to reconcile these considerations and establish the clinical scope of MM.
It is unknown how receptive patients would be to learning MM around the time of surgery. Patients may be sceptical of or uninformed about mind-body therapies [17]. It is also unclear if the religious connotations associated with MM would promote or hinder patient participation [17]. Some patients may be discouraged by anything resembling a religious practice or indeed the opposite may be true [17]. Such phenomena may be subject to many individual patient factors and could be difficult to predict in the absence of empirical data. Future enquiry may seek to better understand the influence of individual patient preferences and values on MM adherence. It may be reasoned that patient education and evidence-based practice could also help dispel misconceptions about MM therapy and foster its adoption amongst the wider community, but research would be needed to corroborate this.
Since its adoption by Western society, MM has become increasingly used as a clinical tool. With an ageing population and increased demand for surgical interventions, complementary therapies such as MM should be considered. In the surgical setting, MM may reduce pain, anxiety, and distress, improve contentment, psychological state, and recovery time, and could decrease the need for high levels of medication and the risks associated with polypharmacy. Beyond its physiological effects, MM may also benefit those seeking relief from mental and physical stresses encountered during their hospital admission. Further research and development are needed to establish viable standardised treatment programs. Despite the mixed opinions about MM, it is likely that future medical practitioners will regard MM as a powerful therapeutic option in addition to its pharmacological counterpart.
Conflict of interest
None.
Acknowledgements
The authors wish to acknowledge Laurel Mackinnon, PhD, ELS, and Sharon Johnatty, PhD, for their invaluable assistance in editing this article.
[1] Wright J. Psychosomatic Interactions. Int J Adv Couns. 1986;9(1):47-60.
[2] Davidson RJ, Kabat-Zinn J, Schumacher J, Rosenkranz M, Muller D, Santorelli SF et al. Alterations in brain and immune function produced by mindfulness meditation. Psychosom Med. 2003; 65(4):564-70.
[3] Ditto B, Eclache M, Goldman N. Short-term autonomic and cardiovascular effects of mindfulness body scan meditation. Ann Behav Med. 2006;32(3):227-34.
[4] Henderson VP, Clemow L, Massion AO, Hurley T, Druker S, Hébert J. The effects of mindfulness-based stress reduction on psychosocial outcomes and quality of life in early-stage breast cancer patients: a randomized trial. Breast Cancer Res Treat. 2012;131(1):99-109. [5] Zeidan F, Martucci KT, Kraft RA, Gordon NS, Mchaffie JG, Coghill RC. Brain mechanisms supporting the modulation of pain by mindfulness meditation. J Neurosci. 2011;31(14):5540-8.
[6] Bettelli G. Anaesthesia for the elderly outpatient: preoperative assessment and evaluation, anaesthetic technique and postoperative pain management. Curr Opin Anaesthesiol. 2010;23(6):726-31.
[7] Miller JJ, Fletcher K, Kabat-Zinn J. Three-year follow-up and clinical implications of a mindfulness meditation-based stress reduction intervention in the treatment of anxiety disorders. Gen Hosp Psychiat. 1995;17(3),192-200.
[8] May A, Gaser C. Magnetic resonance-based morphometry: a window into structural plasticity of the brain. Curr Opin Neurol. 2006;19:407-11.
[9] Grant JA. Meditative analgesia: The current state of the field. Ann N Y Acad Sci. 2014;1307(1):55-63.
[10] Lutz A, Slagter HA, Dunne JD, Davidson RJ. Attention regulation and monitoring in meditation. Trends Cogn Sci. 2008;12(4):163-9.
[11] Bauer-Wu S. Mindfulness meditation. Oncology. 2010;24(10):36-40.
[12] Baer RA. Mindfulness training as a clinical intervention: a conceptual and empirical review. Clin Psychol Sci Pract. 2003;10(2):125-43.
[13] Kabat-Zinn, J. An outpatient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation: theoretical considerations and preliminary results. Gen Hosp Psychistry. 1982;4:33-47.
[14] Nelson EA, Dowsey MM, Knowles SR, Castle DJ, Salzberg MR, Monshat K et al. Systematic review of the efficacy of pre-surgical mind-body based therapies on post-operative outcome measures. Complement Ther Med. 2013;21(6):697-711.
[15] Grossman P, Nieman,L, Schmidt S, Walach H. Mindfulness-based stress reduction and health benefits. A meta-analysis. Psychosom Res. 2004;57:35-43.
[16] Kristeller JL. Mindfulness meditation. In: P. Lehrer, R.L. Woolfolk, & W.E. Simes. Principles and Practice of Stress Management. 2nd Edition. New York: Guildford Press. 2007;393-427.
[17] Hickey WS. Meditation as medicine: a critique. Cross Curr. 2010;60(2):168-84.
[18] Segal Z, Williams M, Teasdale J. Mindfulness-based cognitive therapy for depression: A new approach to preventing relapse: Book review. Cogn Behav Ther. 2002;31:193-4.
[19] Bowen S, Chawla N, Collins SE, Witkiewitz K, Hsu S, Grow J et al. Mindfulness-based relapse prevention for substance use disorders: a pilot efficacy trial. Subst Abus. 2009;30:295-305.
[20] Mavros MN, Athanasiou S, Gkegkes ID, Polyzos KA, Peppas G, Falagas ME et al. Do psychological variables affect early surgical recovery? PLoS ONE. 2011;6(5):e20306.
[21] Pyati S, Gan TJ. Perioperative pain management. CNS Drugs. 2007;21(3):185-211.
[22] Hawker GA, Badley EM, Croxford R, Coyte PH, Glazier RJ, Guan JI et al. A population-based nested case-control study of the costs of hip and knee replacement surgery. Med Care. 2009;47(7):732-41.
[23] Lin PC. An evaluation of the effectiveness of relaxation therapy for patients receiving joint-replacement surgery. J Clin Nurs. 2012;21(5-6):601-8.
[24] Broadbent E, Kahokehr A, Booth RJ, Thomas J, Windsor JA, Buchanan CM et al. A brief relaxation intervention reduces stress and improves surgical wound healing response: A randomised trial. Brain Behav Immun. 2012;26(2):212-7.
[25] Spiller R, Aziz Q, Creed F, Emmanuel A, Houghton L, Hungin P et al. Guidelines on the irritable bowel syndrome: mechanisms and practical management. Gut. 2007;56(12):1770-98.
[26] Palmaro A, Fuzier R, Serres I, Bourrel R, Lapeyre-Mestre M. Analgesic drug consumption increases after carpal tunnel surgery: a pharmacoepidemiological study investigating postoperative pain. Clin Ther. 2015;37(8):e64-5.
[27] Weiser TG, Regenbogen SE, Thompson KD, Haynes AB, Lipsitz SR, Berry WR et al. An estimation of the global volume of surgery: a modelling strategy based on available data. Lancet. 2008;372(9633):139-44.
[28] Wasi P. Brain and meditation. J Neurol Sci. 2009;285(1):s36.
[29] Farb NA, Segal ZV, Mayberg H, Bean J, McKeon D, Fatima Z et al. Attending to the present: mindfulness meditation reveals distinct neural modes of self-reference. Soc Cogn Affect Neur. 2007;2(4):313-322.
[30] Hoge EA, Metcalf CA, Morris LK, Simon NM. Mindfulness meditation for generalized anxiety disorder: effects on resilience, anxiety, and hypothalamic pituitary adrenal (HPA) axis. Psychosom Med. 2013;75(3):A-2.
[31] Kahokehr A, Broadbent E, Wheeler BRL, Sammour T, Hill AG. The effect of perioperative psychological intervention on fatigue after laparoscopic cholecystectomy: a randomized controlled trial. Surg Endosc. 2012;26(6):1730-6.
[32] Wager TD, Scott DJ, Zubiet, J-K. Placebo effects on human mu-opioid activity during pain. Proc Natl Acad Sci USA. 2007;104(26):11056-61.
[33] Tracey I, Ploghaus A, Gati JS, Smith S, Matthews PM, Gati JS et al. Imaging attentional modulation of pain in the periaqueductal gray in humans. J Neurosci. 2002;22(7);2748-52.
[34] Zeidan, F, Adler-Neal AL, Wells RE, Stagnaro E, May LM, Eisenach JC et al. Mindfulness-meditation-based pain relief is not mediated by endogenous opioids. J Neurosci. 2016;36(11):3391-7.
[35] Sprenger C, Eippert F, Finsterbusch J, Bingel U, Rose M, Büchel C. Attention modulates spinal cord responses to pain. Curr Biol. 2012; 22(11):1019-22.
[36] Eippert F, Bingel U, Schoell ED, Yacubian J, Klinger R, Lorenz J et al. Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron. 2009;63(4):533-43.
[37] King CD, Goodin B, Kindler LL, Caudle R, Edwards R, Gravenstein N et al. Reduction of conditioned pain modulation in humans by naltrexone: an exploratory study of the effects of pain catastrophizing. J Behav Med. 2013;36(3):315-27.
[38] Zikopoulos B, Barbas H. Circuits for multisensory integration and attentional modulation through the prefrontal cortex and the thalamic reticular nucleus in primates. Rev Neurosci. 2007;18(6):417-38.
[39] Manyande A, Berg S, Gettins D, Stanford SC, Mazhero S, Marks DF et al. Preoperative rehearsal of active coping imagery influences subjective and hormonal responses to abdominal surgery. Psychosom Med. 1995;57(2):177-82.
[40] Lin P-C. An evaluation of the effectiveness of relaxation therapy for patients receiving joint replacement surgery. J Clin Nurs. 2012;21(5-6):601-8.
[41] Jawaid M, Mushtaq A, Mukhtar S, Khan Z. Preoperative anxiety before elective surgery. Neurosciences. 2007;12(2):145-8.
[42] Manyande A, Salmon P. Effects of pre-operative relaxation on post-operative analgesia: immediate increase and delayed reduction. Br J Health Psychol. 1998;3(Pt 3):215-24.
[43] Scott LE, Clum GA. Examining the interaction effects of coping style and brief interventions in the treatment of postsurgical pain. Pain 1984;20(3):279-91.

Every day medical students and doctors are faced with challenging, ethical, and moral dilemmas. Caring for patients can be draining and bearing witness to their suffering can often take a toll on the mental and emotional health of practitioners. A key psychological component affecting how we react to these situations is empathy. Here, the effects of empathy on our health and relationships with patients as well as the benefits and challenges of using empathic practice are examined.
“Not even one’s own pain weighs so heavy as the pain one feels with someone, for someone, a pain intensified by the imagination and prolonged by a hundred echoes.”
― Milan Kundera, The Unbearable Lightness of Being [1]
Several times a day, if not more often, I see prescriptions for metformin on patient charts. Diabetes in hospital patients is almost as common as perfectionism in medical students; over 900,000 hospitalisations – or 9% of all hospitalisations – in Australia in 2013 were for management of diabetes as the principal or additional diagnosis [2]. Having lived with type 1 diabetes now for over 16 years, I have heard innumerable lectures on the pitfalls of chronic hyperglycaemia. With my last HbA1c falling in the “dangerously high” region at 12.9%, I am all too aware of how this can affect my long-term health. Yet, I cannot in good conscience stand at the bedside of patients with diabetes and lecture them on adherence to medication or better sleeping and eating habits when I myself struggle everyday with poor results. I often find myself torn between judging patients – and myself – for poor control, or letting poor control slide in acceptance of the human capacity for error. Rather than simply ruminating on my own shortcomings, the aim of this essay is to use my own as well as other patients’ experiences to highlight the all-too-real dilemma of allowing empathy to guide us while still separating personal feelings from professional agendas in medicine.
Empathy is a complex phenomenon, involving cognitive and affective processes that affect our capacity to understand and respond to other people’s emotional and mental states. Cognitive empathy can be defined as the awareness and understanding of another’s emotion. Affective empathy refers to the vicarious experience of emotions consistent with those of the observed person and often results in empathic concern, which involves feelings of compassion or concern for another. A more problematic form of affective empathy is personal distress: personal feelings of discomfort and anxiety in response to another’s suffering [3].
A recent popular article published in Scientific American explored the idea of empathy as being a double-edged sword [4]. The authors discussed the psychological construct of empathy’s ability to overwhelm their clinical judgment, however they also underlined its importance in relating to patients and being a well-adjusted human being. The article concluded, “[the] key is knowing when empathy is called for and when it is detrimental. It should not be the goal of physicians, then, to be more empathetic. They should aim instead to find the right balance, the golden mean that optimises care.”
Several studies have demonstrated that as clinical reasoning and experience in medicine widens, empathy decreases [5,6]. Reasons for this change are uncertain, however I question whether the inverse relationship between experience and empathy may be linked to the x-axis of time: the longer medical students spend exposed to the realities of medicine, the less able they become to expose their emotions to the harsh realities of patients’ lives. We can’t save everyone and often we can’t even eliminate much of their burden of disease – so losing the ability to empathise so as to limit emotional and psychological burden is likely a factor here as well. The evidence for this decrease in empathy over time is elegantly demonstrated in a study by Newton and colleagues which revealed that medical students’ empathy scores drop significantly between their first and third years [5]. This study used a standardised empathy scale to evaluate the same class of students every year between first and fourth year, and they found overall medical education was a determinant differentially affecting the vicarious empathy of students, with the greatest impact on male surgical specialties. The authors concluded, “the significant decrease in vicarious empathy is of concern, because empathy is crucial for a successful physician–patient relationship.” Another study of American medical students demonstrated the drop in empathy scores to be most significant across the third year (their first clinical year), with no significant drop during basic sciences teaching [6]. They also reported greater feelings of psychological distress in students over this same period, which is consistent with Australian statistics from Beyond Blue that report one in five medical students have had suicidal thoughts in the past year [7].
While it is undoubtedly true that empathy is necessary for healthy doctor-patient relationships, I question whether there is an element of self-preservation involved in the gradual loss of empathy over the course of our clinical years. Throughout my childhood, my younger sister was in and out of hospital for neurosurgeries involving a hard-to-access cyst in the pineal recess of her third ventricle. I was able to recite these words as a nine-year-old, and as a ten-year-old, I decided I wanted to be a doctor so that I could fix people like her. The problem was that I also hated hospitals; a normally well-mannered child, I would become hysterical after going to see her. In hindsight, I think that paediatric neurosurgery wards do this to a lot of people and in my case this was certainly caused by a vicarious empathetic response of personal distress. The immense suffering you see on these wards can make a bright day seem sombre, and it takes a special kind of nurse and surgeon to work in that environment day-in-day-out. If these people had not distanced themselves from their patients to a degree, the suffering they witnessed would almost certainly cause significant psychological distress. To preserve the emotional well-being of the medical staff on such wards, coping strategies such as intellectualisation, humour, and team support are essential [8].
There was one moment of kindness in that hospital which remains etched in my mind to this day. My sister was a bright child, and on the day before her surgery, unbeknownst to any of us, she secretly wrote a letter outlining her fears and questions for her surgeon. This man was the extremely busy head of neurosurgery and that morning, as usual, he charged into her room for rounds with his trailing procession of residents hanging on every word. After he had checked her over and turned to leave, my sister in a tiny voice announced she had something for him and thrust a piece of coloured paper at him. It was her list, carefully written out in crayon, of questions she wanted answered, number one being “Am I going to die?” He took it from her hand, glanced quickly at it, frowned, and left the room. My mother was appalled at his perceived indifference, while my father tried to soothe the situation with platitudes about how busy the man was. My sister was quiet and said little. Half an hour later we were surprised by the return of the surgeon, this time alone and with his white coat thrown over his shoulder. He walked in, nodded at my mother, and said to my sister, “Now that we’ve gotten rid of all those yucky doctors, let’s take a look at this list.” For the next ten minutes, he carefully went through each question with her and he told her the truth about everything. She calmly listened, occasionally asking more and when finished he rubbed his hands together and asked, “Are we good to go?” After she nodded, he smiled towards my parents and me and strode out of the room. Whether or not he was motivated by empathy I can only speculate, but it seems likely the surgeon recognised the suffering of my parents and sister and he demonstrated empathic concern: sympathy and compassion for others in response to their suffering. Whatever the case, I am thankful that this man was able to control his emotions without losing his humanity and I can only aspire to one day be able to do so as well.
I will conclude by making a case for using empathy in medicine. Empathy is derived from humanity and according to Hippocrates, “Wherever the art of medicine is loved, there is also a love of humanity” [9]. When a patient feels comfortable with a doctor, they are more likely to come forward with their true feelings and admit to forgetting to take prescription medications or to having sex without a condom, whatever the case may be. It is true that as future doctors we need to protect ourselves from feeling too deeply, but if we forget to open our hearts to the people we aim to help, we will risk losing their confidence altogether. Additionally, quite apart from the physician’s need to take a patient’s history to understand their affliction, the process of telling one’s story can be therapeutic for patients [10] and may help facilitate the healing process. Finally, empathy is beneficial to physicians – other physicians have noted that doctors who are more attuned to the psychosocial needs of their patients are less likely to experience burnout [11].
As for myself, I no longer fear going into the hospital but there are still many days where, as a result of connecting with a patient, I feel the urge to cry on my walk home. I try to balance this by looking forward to the time when, as a doctor, I can improve patients’ lives, just as the neurosurgeon did for my sister. I believe that empathy is a good tool to improve listening and understanding of the patient’s perspective. Ultimately my goal is to have the attributes of an excellent physician and a compassionate human being without letting my awareness of the pain of others pain destroy my soul.
Conflicts of interest
None declared.
References
[1] Kundera M. The unbearable lightness of being. New York: Harper & Row; 1984. 38 p.
[2] Diabetes (AIHW) [Internet]. Aihw.gov.au. 2016 [cited 2016 5 Jul]. Available from: http://www.aihw.gov.au/diabetes/
[3] Davis, M. H. Measuring individual differences in empathy: Evidence for a multidimensional approach. J Per Soc Psychol. 1983;44: 113–126.
[4] Haque OS, Waytz A. Why doctors should be more empathetic – but not too much more. Sci Am[Internet]. 2011 Apr 25 [cited 2016 7 Jul]. Available from: http://www.scientificamerican.com/article/doctors-and-dehumanization-effect/
[5] Newton BW, Barber L, Clardy J, Cleveland E, O’Sullivan P. Is there hardening of the heart during medical school? Acad Med. 2008;83(3):244–9.
[6] Hojat M, Vergare M, Maxwell K, Brainard G, Herrine S, Isenberg G et al. The Devil is in the Third Year: A Longitudinal Study of Erosion of Empathy in Medical School. Acad Med. 2009;84(9):1182-1191.
[7] Urgent action needed to improve the mental health and save the lives of Australian doctors and medical students [Internet]. Beyondblue.org.au. 2016 [cited 5 Jul 2016]. Available from: https://www.beyondblue.org.au/docs/default-source/media-release-pdf/urgent-action-needed-to-improve-the-mental-health-and-save-the-lives-of-australian-doctors-and-medical-students-october-2013.pdf?sfvrsn=0
[8] Meadors P, Lamson A. Compassion fatigue and secondary traumatization: Provider self care on intensive care units for children. J Pediatr Health Care. 2008;22(1) 24–34.
[9] Khan Z. Airway management. 1st ed. New York: Springer International Publishing; 2014. 5 p.
[10] Adler HM. The history of the present illness as treatment: who’s listening, and why does it matter? J Am Board Fam Pract. 1997;10(1):28-35.
[11] Anfossi M, Numico G. Empathy in the doctor-patient relationship. J Clin Oncol. 2004;22(11):2258-2259.